Curcumin-loaded nanoparticles reversed radiotherapy-triggered enhancement of MDR1 expression of CNE-2 cells in nasopharyngeal carcinoma
-
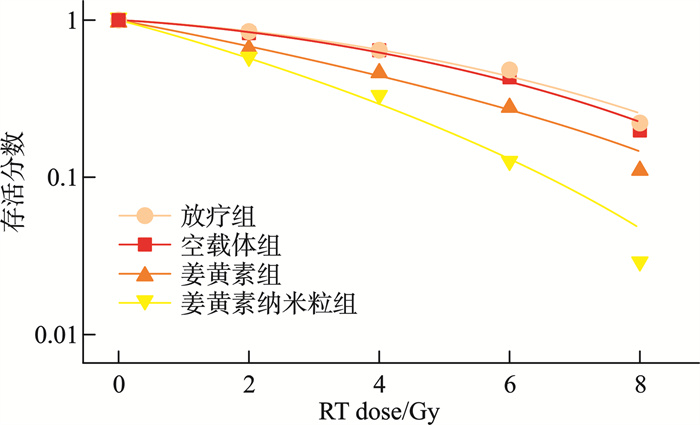
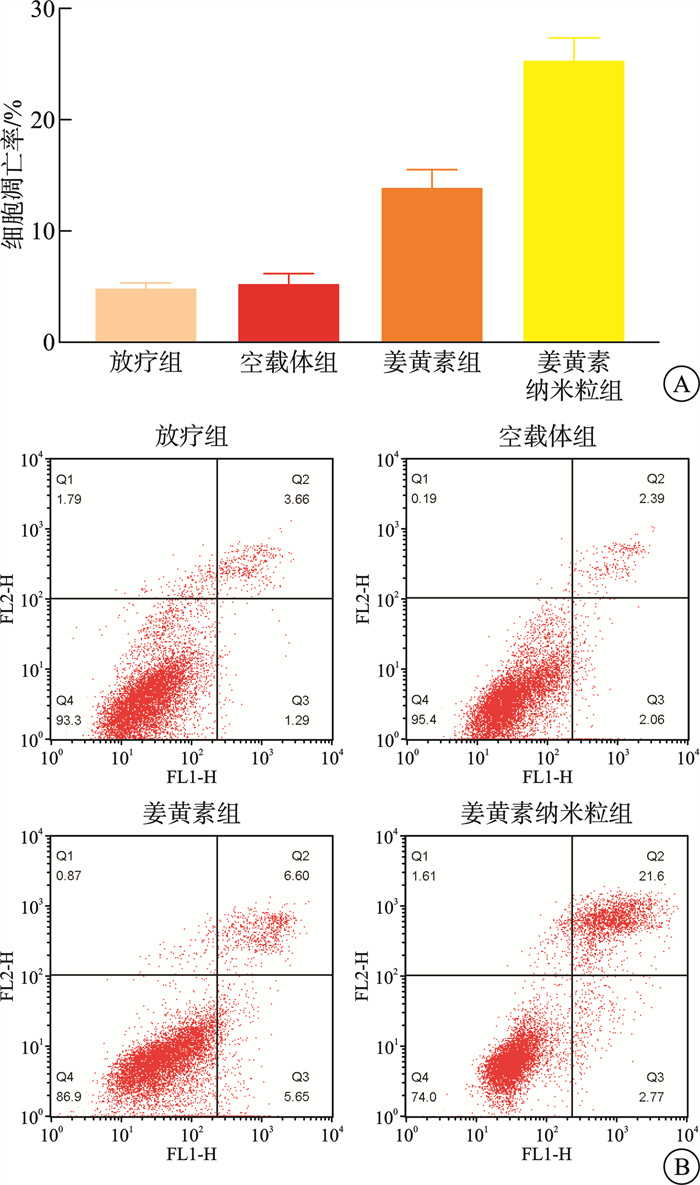
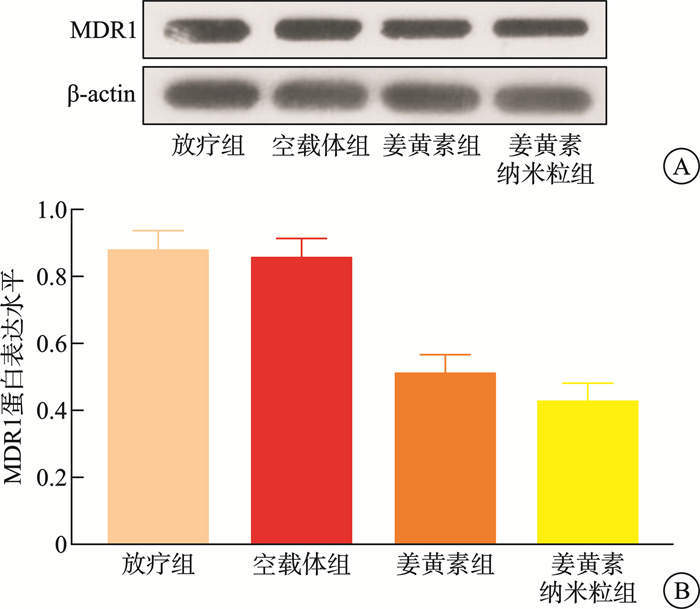
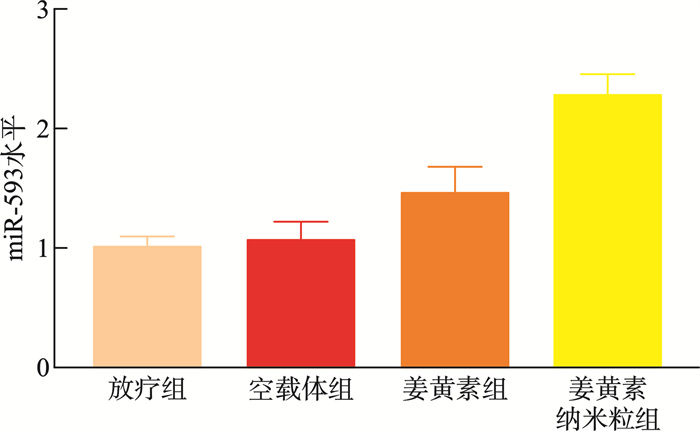
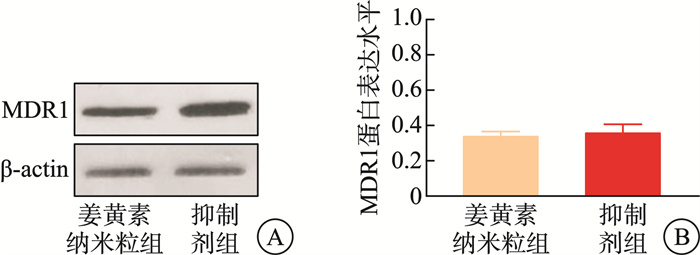
摘要: 目的 本研究将探讨纳米颗粒包封姜黄素对人低分化鼻咽癌细胞系(CNE-2)中强表达多药耐药基因1(multidrug resistance gene 1,MDR1)的影响。 方法 制备姜黄素/壳聚糖-脱氧胆酸纳米颗粒,鼻咽癌细胞接受单纯放疗、空载体、姜黄素和负载姜黄素纳米颗粒的不同处理,然后使用克隆生成测定、细胞凋亡、MDR1和miR-593水平分析细胞存活率。 结果 姜黄素组和负载姜黄素纳米颗粒组的细胞存活分数显著降低。与放疗比较,在接受姜黄素或姜黄素负载纳米颗粒处理的细胞中观察到更高的细胞凋亡率。姜黄素组和负载姜黄素的纳米颗粒组的MDR1水平降低,纳米颗粒组中MDR1表达进一步降低(P<0.05)。在姜黄素组和负载姜黄素的纳米颗粒组中观察到较高的miR-593表达,并且在纳米颗粒组中观察到相对较高的水平(P<0.05)。包裹在纳米颗粒中的姜黄素表现出更强的放疗敏化作用。其与放疗合用可有效抑制鼻咽癌肿瘤生长,抑制MDR1表达,同时增强miR-593。在抑制miR-593后,MDR1表达增强。负载姜黄素的纳米颗粒的放射敏化作用由miR-593调节,但不受MDR1触发。 结论 负载姜黄素的纳米颗粒介导了miR-593的表达增强,进而抑制了MDR1基因的转录和翻译,从而降低了鼻咽癌的放疗抵抗,更有效地抑制了鼻咽癌的生长。
-
关键词:
- 负载姜黄素的纳米颗粒 /
- 鼻咽癌 /
- 多药耐药基因1 /
- miR-593 /
- CNE-2细胞
Abstract: Objective This study explored the effect of nanoparticle-encapsulated curcumin on the highly expressed multidrug resistance gene 1 (MDR1) in a human low-differentiated nasopharyngeal carcinoma cell line (CNE2). Methods Curcumin/chitosan deoxycholic acid nanoparticles were prepared, and the cells were subjected to different treatments: radiotherapy, empty carriers, curcumin, and curcumin-loaded nanoparticles. Cell survival was analyzed using the clonogenic assay, and assessments of apoptosis, MDR1 levels, and miR593 levels were conducted. Results The cell survival fractions in the curcumin group and the curcumin-loaded nanoparticles group were significantly reduced. Notably, higher apoptosis rates were observed in cells treated with curcumin or curcumin-loaded nanoparticles compared to those that received only radiotherapy. Moreover, a decreased MDR1 level was noted in both the curcumin group and the curcumin-loaded nanoparticles group, with further reduction in MDR1 expression observed in the nanoparticle group (P < 0.05). Enhanced expression of miR593 was found in the curcumin group and the curcumin-loaded nanoparticles group, with a relatively higher level in the nanoparticle group (P < 0.05). Curcumin encapsulated in nanoparticles exhibited a stronger radiosensitizing effect. The combination of curcumin and radiotherapy effectively inhibited nasopharyngeal carcinoma (NPC) tumor growth, suppressed MDR1 expression, and enhanced miR593 levels. After inhibiting miR593, MDR1 expression increased. The radiosensitizing effect of curcumin-loaded nanoparticles was regulated by miR593 rather than being triggered by MDR1. Conclusion Curcumin-loaded nanoparticles mediated enhanced expression of miR593, which in turn inhibited the transcription and translation of the MDR1 gene, thereby reducing the radioresistance of NPC and effectively restraining its growth.-
Key words:
- curcumin-loaded nanoparticles /
- nasopharyngeal carcinoma /
- MDR1 /
- miR-593 /
- CNE-2 Cells
-

-
表 1 引物序列
引物 序列 miR-593 正向引物:TGTCTCTGCTGGGGTTTCT 反向引物:GTGCAGGGTCCGAGGTATT U6 正向引物:CTCGCTTCGGCAGCACA 反向引物:AACGCTTCACGAATTTGCGT -
[1] Chang ET, Ye WM, Zeng YX, et al. The evolving epidemiology of nasopharyngeal carcinoma[J]. Cancer Epidemiol Biomarkers Prev, 2021, 30(6): 1035-1047.
[2] Hau PM, Lung HL, Wu M, et al. Targeting Epstein-Barr virus in nasopharyngeal carcinoma[J]. Front Oncol, 2020, 10: 600.
[3] 兰桂萍, 翁敬锦, 李敏, 等. 鼻咽癌放疗后放射性颅底坏死的临床分型分期及治疗策略[J]. 临床耳鼻咽喉头颈外科杂志, 2024, 38(6): 490-495. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2024.06.007
[4] Tang LQ, Lu TY, Li Y, et al. Patterns of failure and survival trends of 720 patients with stage I nasopharyngeal carcinoma diagnosed from 1990-2012: a large-scale retrospective cohort study[J]. J Cancer, 2018, 9(7): 1308-1317.
[5] Wei WI, Kwong DLW. Current management strategy of nasopharyngeal carcinoma[J]. Clin Exp Otorhinolaryngol, 2010, 3(1): 1-12.
[6] He HC, Lin KY, Su Y, et al. Overexpression of β-catenin decreases the radiosensitivity of human nasopharyngeal carcinoma CNE-2 cells[J]. Cell Physiol Biochem, 2018, 50(5): 1929-1944.
[7] Alzoubi KH, Khabour OF, Al-Azzam SI, et al. The role of Multidrug Resistance-1(MDR1) variants in response to atorvastatin among Jordanians[J]. Cytotechnology, 2015, 67(2): 267-274. doi: 10.1007/s10616-013-9682-z
[8] Calado RT, Falcão RP, Garcia AB, et al. Influence of functional MDR1 gene polymorphisms on P-glycoprotein activity in CD34+ hematopoietic stem cells[J]. Haematologica, 2002, 87(6): 564-568.
[9] Wang H, Peng R, Wang JJ, et al. Circulating microRNAs as potential cancer biomarkers: the advantage and disadvantage[J]. Clin Epigenetics, 2018, 10: 59. doi: 10.1186/s13148-018-0492-1
[10] Ma JZ, Zhu YP, Wang Z, et al. miR-593 inhibits proliferation of colon cancer cells in vitro by down-regulating PLK1[J]. Nan Fang Yi Ke Da Xue Xue Bao, 2019, 39(2): 144-149.
[11] Wang QR, Fan HN, Liu Y, et al. Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding RNAs[J]. Int J Oncol, 2014, 44(3): 858-864. doi: 10.3892/ijo.2013.2237
[12] Wong TS, Chan WS, Li CH, et al. Curcumin alters the migratory phenotype of nasopharyngeal carcinoma cells through up-regulation of E-cadherin[J]. Anticancer Res, 2010, 30(7): 2851-2856.
[13] Feng SY, Wang Y, Zhang RK, et al. Curcumin exerts its antitumor activity through regulation of miR-7/Skp2/p21 in nasopharyngeal carcinoma cells[J]. Onco Targets Ther, 2017, 10: 2377-2388.
[14] Yavarpour-Bali H, Ghasemi-Kasman M, Pirzadeh M. Curcumin-loaded nanoparticles: a novel therapeutic strategy in treatment of central nervous system disorders[J]. Int J Nanomedicine, 2019, 14: 4449-4460. doi: 10.2147/IJN.S208332
[15] Liu LM, Yang JM, Ji WG, et al. Curcumin inhibits proliferation of Epstein-Barr virus-associated human nasopharyngeal carcinoma cells by inhibiting EBV nuclear antigen 1 expression[J]. Biomed Res Int, 2019, 2019: 8592921.
[16] Liu ZG, Xu CY, Jiang R, et al. Treatment of locally advanced nasopharyngeal carcinoma by helical tomotherapy: an observational, prospective analysis[J]. Transl Oncol, 2019, 12(5): 757-763.
[17] Ma XK, Zhou JY, Liu JY, et al. LncRNA ANCR promotes proliferation and radiation resistance of nasopharyngeal carcinoma by inhibiting PTEN expression[J]. Onco Targets Ther, 2018, 11: 8399-8408.
[18] Lin JC, Jan JS, Hsu CY, et al. Phase Ⅲ study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival[J]. J Clin Oncol, 2003, 21(4): 631-637. doi: 10.1200/JCO.2003.06.158
[19] Liu Y, Sun HM, Makabel B, et al. The targeting of non-coding RNAs by curcumin: Facts and hopes for cancer therapy(Review)[J]. Oncol Rep, 2019, 42(1): 20-34.
[20] Chainoglou E, Hadjipavlou-Litina D. Curcumin analogues and derivatives with anti-proliferative and anti-inflammatory activity: Structural characteristics and molecular targets[J]. Expert Opin Drug Discov, 2019, 14(8): 821-842.
[21] Sandur SK, Deorukhkar A, Pandey MK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity[J]. Int J Radiat Oncol Biol Phys, 2009, 75(2): 534-542.
[22] Dei Cas M, Ghidoni R. Dietary curcumin: correlation between bioavailability and health potential[J]. Nutrients, 2019, 11(9): 2147.
[23] Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation[J]. Biomaterials, 2010, 31(25): 6597-6611.
[24] Navya PN, Kaphle A, Srinivas SP, et al. Current trends and challenges in cancer management and therapy using designer nanomaterials[J]. Nano Converg, 2019, 6(1): 23.
[25] Hatcher H, Planalp R, Cho J, et al. Curcumin: from ancient medicine to current clinical trials[J]. Cell Mol Life Sci, 2008, 65(11): 1631-1652.
[26] Fergusson JR, Ussher JE, Kurioka A, et al. High MDR-1 expression by MAIT cells confers resistance to cytotoxic but not immunosuppressive MDR-1 substrates[J]. Clin Exp Immunol, 2018, 194(2): 180-191.
[27] Fan HN, Shao M, Huang SH, et al. MiR-593 mediates curcumin-induced radiosensitization of nasopharyngeal carcinoma cells via MDR1[J]. Oncol Lett, 2016, 11(6): 3729-3734.
-

计量
- 文章访问数: 155
- 施引文献: 0



 下载:
下载: