Changes in intestinal flora associated with childhood sleep-disordered breathing and the pathogenesis of non-alcoholic fatty liver disease in children
-
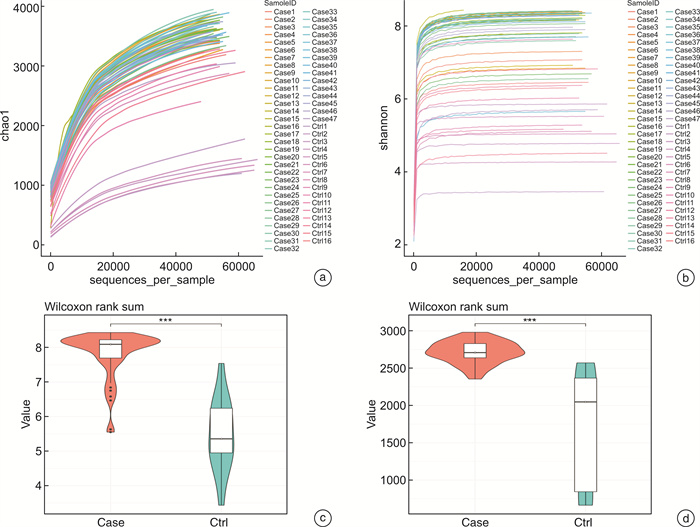
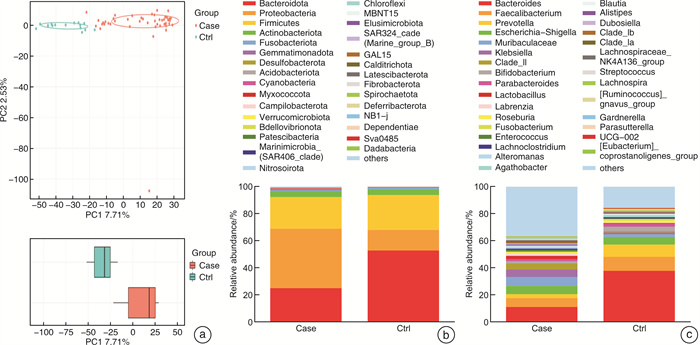
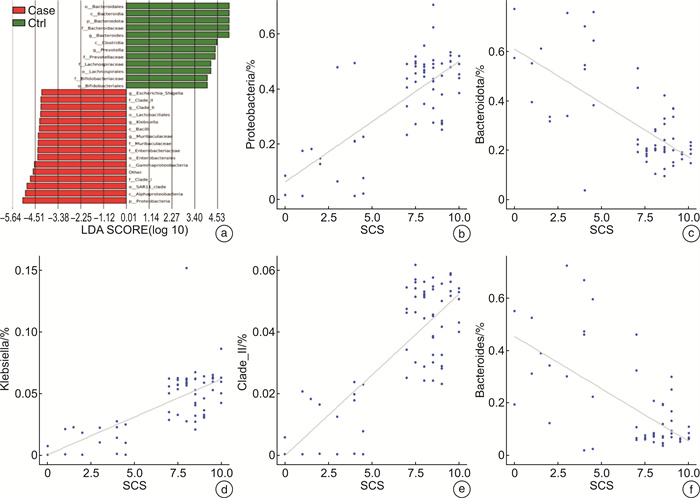
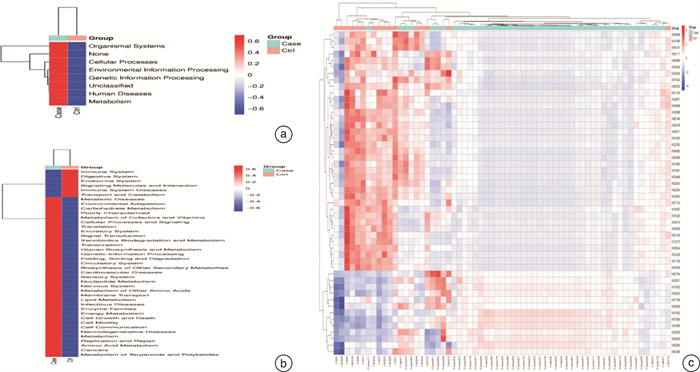
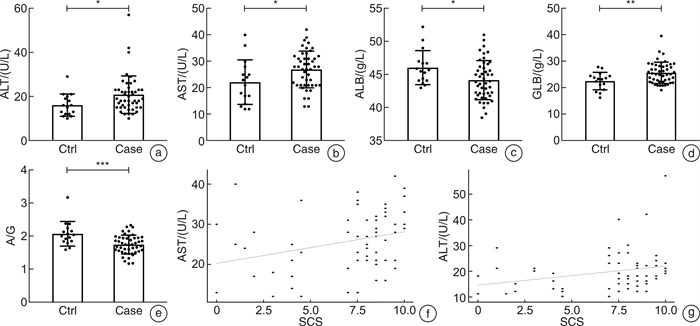
摘要: 目的 探讨儿童睡眠呼吸障碍(sleep-disordered breathing,SDB)、肠道菌群与儿童非酒精性脂肪肝病(non-alcoholic fatty liver disease,NAFLD)之间的相互作用。 方法 共纳入63例非肥胖儿童(试验组47例合并SDB,对照组16例未合并SDB的)参与本研究。分别评估2组儿童的肝功能状态和SDB程度。采用高通量16S rRNA测序来检测2组儿童的肠道菌群组成和功能变化。 结果 与对照组比较,试验组儿童的血清谷草转氨酶、谷丙转氨酶增高;肠道菌群的变形菌门相对丰度增加,拟杆菌门相对丰度显著减少;肠道菌群的膜运输、碳水化合物代谢、脂质代谢等功能富集。 结论 2组儿童肝功能状态和肠道菌群的组成、功能注释存在显著差异。儿童SDB相关肠道菌群的变化可能与儿童NAFLD的发病机制相关。
-
关键词:
- 儿童睡眠呼吸障碍 /
- 儿童非酒精性脂肪肝病 /
- 肠道菌群
Abstract: Objective To explore the interaction between pediatric sleep-disordered breathing(SDB), the intestinal microbiota, and pediatric non-alcoholic fatty liver disease(NAFLD). Methods A total of 63 non-obese children(47 children with SDB in the experimental group and 16 without SDB in the control group) were enrolled in this study. The liver function and degree of SDB were assessed in both groups. High-throughput 16S rRNA sequencing was conducted to detect the composition and functional variations of the intestinal microbiota in the two groups of children. Results Compared with children in the experimental group, serum ALT and AST levels were higher in the control group. and the relative proteobacteria abundance of intestinal flora increased, and the relative abundance of Bacteroidetes decreased significantly. Function including membrane transport, carbohydrate metabolism and lipid metabolism, were enriched in the intestinal microbiota of children with SDB. Conclusion The composition and functional annotation of the pediatric liver functional status and gut microbiota were significantly different between the two groups of children with and without SDB. Changes in SDB-associated intestinal bacterial abundance may be related to the pathogenesis of pediatric NAFLD. -

-
表 1 2组儿童临床基本资料
项目 试验组 对照组 P 例数 47 16 — 性别/例 0.720 男 24 7 女 23 9 年龄/岁 6.09±2.70 6.13±3.78 0.964 体重指数 17.17±2.79 17.68±2.92 0.532 SCS 8.45±0.91 2.69±1.62 <0.001 -
[1] Bue AL, Salvaggio A, Insalaco G. Obstructive sleep apnea in developmental age. A narrative review[J]. Eur J Pediatr, 2020, 179(3): 357-365. doi: 10.1007/s00431-019-03557-8
[2] Franzosa EA, Sirota-Madi A, Avila-Pacheco J, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease[J]. Nat Microbiol, 2019, 4: 293-305.
[3] Zhou YJ, Jackson D, Bacharier LB, et al. The upper-airway microbiota and loss of asthma control among asthmatic children[J]. Nat Commun, 2019, 10(1): 5714. doi: 10.1038/s41467-019-13698-x
[4] Siljander H, Honkanen J, Knip M. Microbiome and type 1 diabetes[J]. EBio Medicine, 2019, 46: 512-521.
[5] Collado MC, Katila MK, Vuorela NM, et al. Dysbiosis in snoring children: an interlink to comorbidities?[J]. J Pediatr Gastroenterol Nutr, 2019, 68(2): 272-277. doi: 10.1097/MPG.0000000000002161
[6] Valentini F, Evangelisti M, Arpinelli M, et al. Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study[J]. Sleep Med, 2020, 76: 140-147. doi: 10.1016/j.sleep.2020.10.017
[7] Song PG, Yu JY, Wang ML, et al. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese children[J]. Int J Environ Res Public Health, 2017, 14(5): 465. doi: 10.3390/ijerph14050465
[8] European Association for the Study of the Liver(EASL), European Association for the Study of Diabetes(EASD), European Association for the Study of Obesity(EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease[J]. Diabetologia, 2016, 59(6): 1121-1140. doi: 10.1007/s00125-016-3902-y
[9] Chen LD, Chen MX, Chen GP, et al. Association between obstructive sleep apnea and non-alcoholic fatty liver disease in pediatric patients: a meta-analysis[J]. Pediatr Obes, 2021, 16(3): e12718. doi: 10.1111/ijpo.12718
[10] Villa MP, Paolino MC, Castaldo R, et al. Sleep clinical record: an aid to rapid and accurate diagnosis of paediatric sleep disordered breathing[J]. Eur Respir J, 2013, 41(6): 1355-1361. doi: 10.1183/09031936.00215411
[11] Vanek J, Prasko J, Genzor S, et al. Obstructive sleep apnea, depression and cognitive impairment[J]. Sleep Med, 2020, 72: 50-58. doi: 10.1016/j.sleep.2020.03.017
[12] Bergler H, Fuchsbichler S, Högenauer G, et al. The enoyl-[acyl-carrier-protein]reductase(FabI)of Escherichia coli, which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA[J]. Eur J Biochem, 1996, 242(3): 689-694. doi: 10.1111/j.1432-1033.1996.0689r.x
[13] Vos MB, Abrams SH, Barlow SE, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children[J]. J Pediatr Gastroenterol Nutr, 2017, 64(2): 319-334. doi: 10.1097/MPG.0000000000001482
[14] 邓哲, 王桂香, 侯钰玮, 等. 出生体重与儿童阻塞性睡眠呼吸暂停关系的研究[J]. 临床耳鼻咽喉头颈外科杂志, 2024, 38(3): 230-234. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2024.03.010
[15] 李晶, 杨屈扬, 许莹, 等. 儿童肥胖与阻塞性睡眠呼吸暂停的相关性研究进展[J]. 临床耳鼻咽喉头颈外科杂志, 2023, 37(4): 318-322. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2023.04.017
[16] Vasques-Monteiro IML, Silva-Veiga FM, Miranda CS, et al. A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice[J]. Nutr Res, 2021, 91: 26-35. doi: 10.1016/j.nutres.2021.04.008
[17] Guo Q, Tang Y, Li Y, et al. Perinatal high-salt diet induces gut microbiota dysbiosis, bile acid homeostasis disbalance, and NAFLD in weanling mice offspring[J]. Nutrients, 2021, 13(7): 2135. doi: 10.3390/nu13072135
[18] Lee HB, Do MH, Jhun H, et al. Amelioration of hepatic steatosis in mice through Bacteroides uniformis CBA7346-mediated regulation of high-fat diet-induced insulin resistance and lipogenesis[J]. Nutrients, 2021, 13(9): 2989. doi: 10.3390/nu13092989
[19] Zhao YZ, Zhou JL, Liu JQ, et al. Metagenome of gut microbiota of children with nonalcoholic fatty liver disease[J]. Front Pediatr, 2019, 7: 518. doi: 10.3389/fped.2019.00518
-




 下载:
下载: