Efficacy of glucocorticoid stent implantation in ethmoid sinus after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps
-
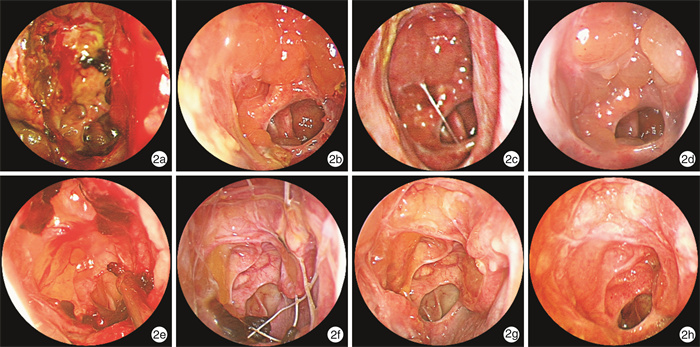
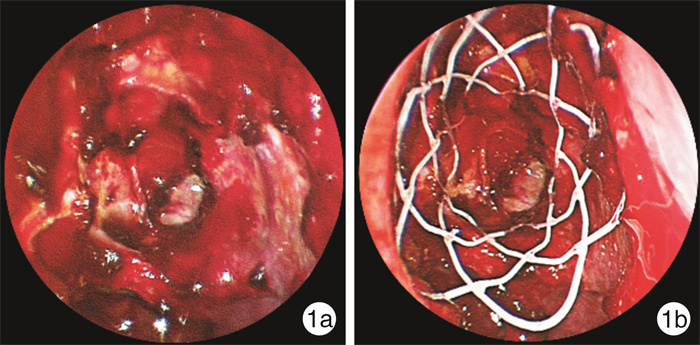
摘要: 目的 评估在功能性内镜鼻窦手术(FESS)术后2周植入糖皮质激素鼻窦支架治疗慢性鼻窦炎伴鼻息肉(CRSwNP)的疗效。方法 选取双侧病变程度相近的49例全组CRSwNP患者,随机分为支架组(25例)和对照组(24例)。支架组患者于FESS术后2周将糖皮质激素鼻窦支架植入双侧筛窦,对照组仅进行术腔清理。2组接受相同的术后治疗,分别于术后2、4、8、12周行随访评估。采集患者的鼻部症状视觉模拟量表(VAS)评分;通过鼻内镜检查评估筛窦阻塞及结痂/凝结物情况、有无囊泡或息肉样变黏膜形成、中鼻甲位置、有无粘连、鼻窦黏膜是否上皮化及筛窦术区是否需要干预等。应用GraphPad Prism 9进行统计学分析。结果 术后4周和8周,支架组患者鼻塞、流涕两项VAS评分较对照组有改善,差异有统计学意义(P < 0.05)。在头面部闷胀感、嗅觉减退及鼻腔干燥/结痂感3项VAS评分中,2组差异无统计学意义(P>0.05)。相较于对照组,支架组患者在术后4、8、12周,筛窦黏膜形成囊泡或息肉样变率更低;术后12周,支架组筛窦腔黏膜上皮化率明显高于对照组;整个随访过程中,支架组需要术后干预的比例明显低于对照组(P < 0.05)。在术后8、12周,支架组中鼻甲外移发生率较低;术后8周,支架组患者术腔粘连率显著降低(P < 0.05)。结论 FESS术后植入糖皮质激素鼻窦支架可以保持窦腔通畅,改善术腔炎症状态,减少术后干预,促进术腔良性转归。Abstract: Objective To evaluate the efficacy of glucocorticoid sinus stents implanted 2 weeks after functional endoscopic sinus surgery(FESS) for the treatment of chronic rhinosinusitis with nasal polyps(CRSwNP).Methods CRSwNP patients with similar bilateral lesions were randomly divided into two groups, with a stent group of 25 patients and a control group of 24 patients. Patients in the stent group had glucocorticoid sinus stents implanted into the bilateral ethmoid sinuses 2 weeks after FESS, while the control group underwent postoperative debridement only. Follow-up assessments occurred at postoperative weeks 2, 4, 8, and 12. Patients were asked to assess their sensation of nasal symptoms using a 10-point visual analog scale. Efficacy was assessed by endoscopic evaluations. Sinus obstruction, crusting/coagulation, polyp formation, middle turbinate position, adhesions, mucosa epithelialization, and postoperative intervention were assessed as efficacy outcomes. GraphPad Prism 9 was applied for statistical analysis.Results At 4 and 8 weeks postoperatively, the stent group showed significant improvement in VAS scores of nasal congestion and runny nose compared with the control group(P < 0.05). No significant difference was observed in the VAS scores of head and facial stuffiness, loss of smell, or nasal dryness/crusting between the two groups(P>0.05). Compared with the control group, the stent group had a lower rate of polypoid formation at 4, 8, and 12 weeks postoperatively. At postoperative week 12, the rate of mucosal epithelialization in the ethmoid cavity was significantly higher in the stent group. During the follow-up, the frequency of postoperative intervention was significantly lower in the stent group than in the control group(P < 0.05). Besides, a lower incidence of middle turbinate lateralization was found in the stent group at 8 and 12 weeks postoperatively. At 8 weeks postoperatively, the stent group had a percentage of adhesion lower than that of the control group(all P < 0.05).Conclusion Implantation of glucocorticoid sinus stents after FESS can maintain sinus cavity patency, improve the inflammatory status of the operative cavity, reduce postoperative interventions, and promote benign regression of the operative cavity.
-
Key words:
- sinusitis /
- nasal polyp /
- endoscopic sinus surgery /
- nasal sinus stent /
- glucocorticoids
-

-
表 1 鼻部症状VAS评分相较于术前变化差值比较
症状 术前 术后2周 术后4周 术后8周 术后12周 鼻塞 支架组 6.00±2.25 -3.92±2.18 -5.34±2.491) -5.22±2.481) -5.20±2.13 对照组 5.81±2.21 -3.63±2.58 -3.38±3.13 -3.71±2.75 -4.50±2.33 流涕 支架组 5.84±2.20 -3.28±2.53 -4.60±2.461) -5.08±1.771) -5.04±2.69 对照组 4.96±2.04 -3.06±2.58 -3.17±2.43 -3.77±2.54 -4.23±2.21 头部闷胀感 支架组 1.54±2.01 -0.72±2.17 -1.28±1.91 -1.30±1.89 -1.32±1.96 对照组 1.42±1.86 -0.52±2.00 -1.04±2.05 -1.21±1.84 -1.25±2.07 嗅觉减退 支架组 5.02±4.15 -0.50±3.31 -1.30±3.98 -1.94±3.71 -2.82±4.21 对照组 4.42±3.97 -0.52±4.02 -1.46±4.90 -2.35±3.76 -2.29±3.63 鼻腔干燥/结痂感 支架组 1.32±1.68 0.24±1.99 0.84±2.17 0.18±2.21 -0.48±2.08 对照组 1.40±1.88 0.08±2.44 -0.13±2.56 -0.40±2.42 -0.79±2.39 与对照组比较,1)P < 0.05。 表 2 鼻内镜检查术腔评估及术后干预率比较
观察指标 术后2周 术后4周 术后8周 术后12周 筛窦阻塞VAS评分 支架组 2.54±1.32 2.84±1.072) 1.96±1.571) 1.06±1.12 对照组 2.46±1.02 4.04±1.30 3.13±1.94 1.40±1.33 筛窦结痂/凝结物VAS评分 支架组 4.76±1.17 3.76±1.231) 1.48±0.96 0.52±0.82 对照组 4.92±1.02 2.92±1.25 1.29±1.04 0.29±0.62 囊泡或息肉样变(2~3分)/例(%) 支架组 2(8.0) 5(20.0)1) 3(12.0)1) 1(4.0)1) 对照组 4(16.7) 13(54.2) 10(41.7) 6(25.0) 筛窦黏膜上皮化/例(%) 支架组 0 3(12.0) 14(56.0) 21(84.0)1) 对照组 0 1(4.2) 8(33.3) 13(54.2) 筛窦术后干预/例(%) 支架组 — 10(40.0)1) 6(24.0)1) 1(4.0)1) 对照组 — 18(75.0) 13(54.2) 7(29.2) 与对照组比较,1)P < 0.05,2)P < 0.01。 表 3 并发症发生情况比较
例(%) 组别 中鼻甲外移(2~3分) 粘连 术后2周 术后4周 术后8周 术后12周 术后2周 术后4周 术后8周 术后12周 支架组 2(8.0) 1(4.0) 0 0 0 0 1(4.0) 2(8.0) 对照组 5(20.8) 3(12.5) 7(29.2) 7(29.2) 1(4.2) 1(4.2) 6(25.0) 7(29.2) P值 0.247 0.349 0.004 0.004 0.490 0.490 0.049 0.074 -
[1] Shi JB, Fu QL, Zhang H, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities[J]. Allergy, 2015, 70(5): 533-539. doi: 10.1111/all.12577
[2] 中华耳鼻咽喉头颈外科杂志编辑委员会鼻科组, 中华医学会耳鼻咽喉头颈外科学分会鼻科学组. 中国慢性鼻窦炎诊断和治疗指南(2018)[J]. 中华耳鼻咽喉头颈外科杂志, 2019, 54(2): 81-100. doi: 10.3760/cma.j.issn.1673-0860.2019.02.001
[3] Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021[J]. Int Forum Allergy Rhinol, 2021, 11(3): 213-739. doi: 10.1002/alr.22741
[4] Schilling AL, Cannon E, Lee SE, et al. Advances in controlled drug delivery to the sinonasal mucosa[J]. Biomaterials, 2022, 282: 121430. doi: 10.1016/j.biomaterials.2022.121430
[5] Han JK, Kern RC. Topical therapies for management of chronic rhinosinusitis: steroid implants[J]. Int Forum Allergy Rhinol, 2019, 9(S1): S22-S26. doi: 10.1002/alr.22344
[6] 倪祎华, 许政敏. 鼻窦球囊扩张结合全降解药物支架治疗儿童慢性鼻窦炎的疗效分析[J]. 临床耳鼻咽喉头颈外科杂志, 2020, 34(6): 481-485, 491. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2020.06.001
[7] Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020[J]. Rhinology, 2020, 58(Suppl S29): 1464.
[8] Wei CC, Kennedy DW. Mometasone implant for chronic rhinosinusitis[J]. Med Devices(Auckl), 2012, 5: 75-80.
[9] 万玉峰, 刘龙生, 徐盼盼, 等. 慢性鼻窦炎内镜术中使用可吸收鼻窦药物支架的疗效分析[J]. 临床耳鼻咽喉头颈外科杂志, 2020, 34(5): 417-420. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2020.05.007
[10] 王奎吉, 娄鸿飞, 王成硕, 等. 嗜酸性粒细胞型鼻息肉内镜术中植入全降解鼻窦药物支架的疗效观察[J]. 中国耳鼻咽喉头颈外科, 2021, 28(4): 199-202. https://www.cnki.com.cn/Article/CJFDTOTAL-EBYT202104002.htm
[11] Huang Z, Zhou B, Wang D, et al. Comparison of Bioabsorbable Steroid-Eluting Sinus Stents Versus Nasopore After Endoscopic Sinus Surgery: A Multicenter, Randomized, Controlled, Single-Blinded Clinical Trial[J]. Ear Nose Throat J, 2022, 101(4): 260-267. doi: 10.1177/0145561320947632
[12] Wang C, Yu L, Chu X, et al. Short-term postoperative efficacy of steroid-eluting stents for eosinophilic chronic rhinosinusitis with nasal polyps: A randomized clinical trial[J]. Int Forum Allergy Rhinol, 2022.
[13] Hopkins C, Browne JP, Slack R, et al. The Lund-Mackay staging system for chronic rhinosinusitis: how is it used and what does it predict?[J]. Otolaryngol Head Neck Surg, 2007, 137(4): 555-561. doi: 10.1016/j.otohns.2007.02.004
[14] Han JK, Forwith KD, Smith TL, et al. RESOLVE: a randomized, controlled, blinded study of bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis[J]. Int Forum Allergy Rhinol, 2014, 4(11): 861-870. doi: 10.1002/alr.21426
[15] Ow R, Groppo E, Clutter D, et al. Steroid-eluting sinus implant for in-office treatment of recurrent polyposis: a pharmacokinetic study[J]. Int Forum Allergy Rhinol, 2014, 4(10): 816-822. doi: 10.1002/alr.21414
[16] Matheny KE, Carter KB Jr, Tseng EY, et al. Safety, feasibility, and efficacy of placement of steroid-eluting bioabsorbable sinus implants in the office setting: a prospective case series[J]. Int Forum Allergy Rhinol, 2014, 4(10): 808-815. doi: 10.1002/alr.21416
[17] Shen J, Welch K, Kern R. Mometasone furoate sinus implant-a new targeted approach to treating recurrent nasal polyp disease[J]. Expert Rev Clin Pharmacol, 2018, 11(12): 1163-1170. doi: 10.1080/17512433.2018.1549485
[18] Luong A, Ow RA, Singh A, et al. Safety and Effectiveness of a Bioabsorbable Steroid-Releasing Implant for the Paranasal Sinus Ostia: A Randomized Clinical Trial[J]. JAMA Otolaryngol Head Neck Surg, 2018, 144(1): 28-35.
[19] 陈建磊, 张思瑶, 王永台, 等. 鼻窦支架植入术治疗嗜酸性粒细胞型慢性鼻-鼻窦炎短期疗效评估[J]. 中国耳鼻咽喉头颈外科, 2021, 28(4): 203-207. https://www.cnki.com.cn/Article/CJFDTOTAL-EBYT202104003.htm
[20] 施晓琼, 唐海红, 郑宏良, 等. 缓释糖皮质激素支架在全组慢性鼻窦炎伴鼻息肉患者额窦口植入中的疗效观察[J]. 中华耳鼻咽喉头颈外科杂志, 2021, 56(8): 824-829.
[21] Schneider AL, Racette SD, Kang AK, et al. Use of intraoperative frontal sinus mometasone-eluting stents decreased interleukin 5 and interleukin 13 in patients with chronic rhinosinusitis with nasal polyps[J]. Int Forum Allergy Rhinol, 2022, 12(11): 1330-1339.
[22] He J, Liu Q, Wang Q, et al. Soluble drug-eluting stent in preventing and treating nasal cavity and sinus adhesion after endoscopic sinus surgery[J]. Asian J Surg, 2022, 45(6): 1287-1288.
[23] Hwang CS, Al Sharhan SS, Kim BR, et al. Randomized controlled trial of steroid-soaked absorbable calcium alginate nasal packing following endoscopic sinus surgery[J]. Laryngoscope, 2018, 128(2): 311-316.
[24] Forwith KD, Han JK, Stolovitzky JP, et al. RESOLVE: bioabsorbable steroid-eluting sinus implants for in-office treatment of recurrent sinonasal polyposis after sinus surgery: 6-month outcomes from a randomized, controlled, blinded study[J]. Int Forum Allergy Rhinol, 2016, 6(6): 573-581.
[25] 邱增玉, 方平, 汪东, 等. 慢性鼻窦炎内镜鼻窦手术中植入全降解鼻窦药物支架有效性及安全性的Meta分析[J]. 医学信息, 2021, 34(13): 75-80. https://www.cnki.com.cn/Article/CJFDTOTAL-YXXX202113020.htm
[26] 陈滨, 王玲, 刘鑫, 等. 慢性鼻窦炎伴鼻息肉鼻内镜手术保留中鼻甲效果观察[J]. 人民军医, 2016, 59(11): 1169-1171. https://www.cnki.com.cn/Article/CJFDTOTAL-RMJZ201611044.htm
[27] Murr AH, Smith TL, Hwang PH, et al. Safety and efficacy of a novel bioabsorbable, steroid-eluting sinus stent[J]. Int Forum Allergy Rhinol, 2011, 1(1): 23-32.
[28] 杨钦泰, 陈建军, 谭国林, 等. 鼻用糖皮质激素治疗变应性鼻炎专家共识(2021, 上海)[J]. 中国耳鼻咽喉颅底外科杂志, 2021, 27(4): 365-371. https://www.cnki.com.cn/Article/CJFDTOTAL-ZEBY202104001.htm
-




 下载:
下载: