-
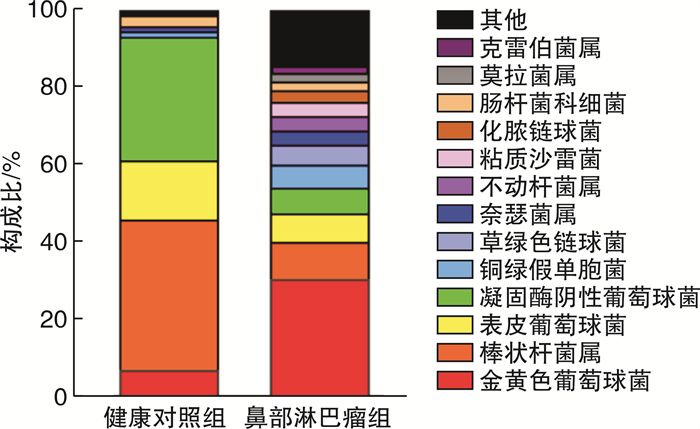
摘要: 目的 探索鼻部淋巴瘤患者鼻腔分泌物中细菌的分布特点。方法 回顾性分析青岛大学附属医院2019年1月—2022年6月就诊的86例鼻部淋巴瘤患者与39例健康体检人员总鼻道的鼻分泌物的细菌培养结果,分析和比较鼻部淋巴瘤与健康人群鼻腔细菌分布的差异。结果 健康人群鼻部细菌以棒状杆菌最多(38.90%),其次为凝固酶阴性葡萄球菌(31.95%)、表皮葡萄球菌(15.28%)、金黄色葡萄球菌(6.95%);而鼻部淋巴瘤患者鼻部细菌分布则以金黄色葡萄球菌最多(30.37%),其次为棒状杆菌(9.63%)、表皮葡萄球菌(7.41%)、凝固酶阴性葡萄球菌(6.67%)。其中81例患者检出细菌,阳性率高达94.19%(81/86)。结论 鼻部淋巴瘤患者鼻分泌物中的细菌分布与健康人相比存在显著差异,其致病菌以金黄色葡萄球菌为主,这为临床上预防和治疗鼻部淋巴瘤或合并感染提供了指导意义。Abstract: Objective To investigate the etiological characteristics of nasal bacterial infection in patients with nasal lymphoma.Methods The results of bacterial culture of nasal secretions from 39 healthy people and 86 patients with nasal lymphoma in the Affiliated Hospital of Qingdao University from January 2019 to June 2022 were retrospectively analyzed, and the differences in nasal bacteria distribution between nasal lymphoma and healthy people were analyzed and compared.Results Corynebacterium(38.90%) was the most common bacteria in the nasal cavity of healthy people, followed by coagulase-negative Staphylococcus(31.95%), Staphylococcus epidermidis(15.28%) and Staphylococcus aureus(6.95%). The most common bacteria in nasal lymphoma patients was Staphylococcus aureus(30.37%), followed by Corynebacterium(9.63%), Staphylococcus epidermidis(7.41%) and coagulase negative Staphylococcus(6.67%). A total of 81 nasal lymphoma patients were detected with bacteria, positive rate is as high as 94.19%(81/86).Conclusion Staphylococcus aureus is the main pathogenic bacteria in nasal secretion of patients with nasal lymphoma, which provides guiding significance for the clinical prevention and treatment of nasal lymphoma complicated with infection or not.
-
Key words:
- lymphoma /
- microbiology /
- staphylococcus aureus /
- infection
-

-
表 1 两组一般临床资料比较
例 组别 例数 性别 年龄/岁 男 女 < 18 18~60 >60 健康对照组 39 10 29 4 35 0 鼻部淋巴瘤组 86 52 34 0 65 21 表 2 两组鼻分泌物细菌培养结果比较
株(%) 细菌种类 健康对照组 鼻部淋巴瘤组 合计 χ2 P值 金黄色葡萄球菌 5(6.95) 41(30.37) 46(22.22) 14.909 < 0.001 棒状杆菌属 28(38.90) 13(9.63) 41(19.81) 25.309 < 0.001 表皮葡萄球菌 11(15.28) 10(7.41) 21(10.15) 3.191 0.074 凝固酶阴性葡萄球菌 23(31.95) 9(6.67) 32(15.46) 22.958 < 0.001 铜绿假单胞菌 1(1.38) 8(5.93) 9(4.35) 2.324 0.127 草绿色链球菌 0 7(5.20) 7(3.40) - - 奈瑟菌属 1(1.38) 5(3.70) 6(2.90) 0.894 0.344 不动杆菌属 0 5(3.70) 5(2.42) - - 粘质沙雷菌 0 5(3.70) 5(2.42) - - 化脓链球菌 0 4(2.97) 4(1.95) - - 肠杆菌科细菌 2(2.78) 3(2.22) 5(2.42) 0.061 0.804 莫拉菌属 0 3(2.22) 3(1.45) - - 克雷伯菌属 0 3(2.22) 3(1.45) - - 合适柠檬酸杆菌 0 1(0.74) 1(0.48) - - 路邓葡萄球菌 0 1(0.74) 1(0.48) - - 恶臭假单胞菌 0 1(0.74) 1(0.48) - - 粪肠球菌 0 1(0.74) 1(0.48) - - 唾液链球菌 0 1(0.74) 1(0.48) - - 近平滑假丝酵母 0 1(0.74) 1(0.48) - - 节杆菌属 0 1(0.74) 1(0.48) - - 乳明串珠菌 0 1(0.74) 1(0.48) - - 噬二氧化碳纤维菌 0 1(0.74) 1(0.48) - - 微球菌 0 1(0.74) 1(0.48) - - 克氏库克菌 0 1(0.74) 1(0.48) - - 奇异变形杆菌 0 1(0.74) 1(0.48) - - 肠球菌 0 1(0.74) 1(0.48) - - 沃氏葡萄球菌 0 1(0.74) 1(0.48) - - 酿脓链球菌 0 1(0.74) 1(0.48) - - 龋齿放线菌 0 1(0.74) 1(0.48) - - 无乳链球菌 0 1(0.74) 1(0.48) - - 类干酪乳杆菌 0 1(0.74) 1(0.48) - - 麻疹孪生球菌 0 1(0.74) 1(0.48) - - 革兰阳性芽孢杆菌 1(1.38) 0 1(0.48) - - 合计 72(100.00) 135(100.00) 207(100.00) - - -
[1] Kwok HM, Ng FH, Chau CM, et al. Multimodality imaging of extra-nodal lymphoma in the head and neck[J]. Clin Radiol, 2022, 77(8): e549-e559. doi: 10.1016/j.crad.2022.04.017
[2] Mugnaini EN, Ghosh N. Lymphoma[J]. Prim Care, 2016, 43(4): 661-675. doi: 10.1016/j.pop.2016.07.012
[3] Eide JG, Kshirsagar RS, Birkenbeuel JL, et al. Primary sinonasal lymphoma: A multi-institutional experience of clinical presentation, treatment, and outcomes[J]. Int Forum Allergy Rhinol, 2022.
[4] 李鑫, 宁丰, 王景文. 鼻结外NK/T细胞淋巴瘤患者鼻部细菌感染的病原学特点及相关指标的研究[J]. 临床和实验医学杂志, 2013, 12(1): 15-17. https://www.cnki.com.cn/Article/CJFDTOTAL-SYLC201301005.htm
[5] 范丹, 郭欢绪, 肖方, 等. PD-1单抗联合培门冬酶治疗复发难治结外NK/T细胞淋巴瘤疗效和安全性的初步分析[J]. 临床血液学杂志, 2022, 35(7): 490-494. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXZ202207008.htm
[6] Polyatskin IL, Artemyeva AS, Krivolapov YA. Peresmotrennaia klassifikatsiia VOZ opukholeǐ gemopoé-ticheskoǐ i limfoidnoǐ tkaneǐ, 2017(4-e izdanie): opukholi limfoidnoǐ tkani[Revised WHO classification of tumors of hematopoietic and lymphoid tissues, 2017(4th edition): lymphoid tumors][J]. Arkh Patol, 2019, 81(3): 59-65. doi: 10.17116/patol20198103159
[7] 靳晶, 许昱. 鼻腔分泌物中生物标志物在慢性鼻窦炎内在型诊断和临床应用中的研究进展[J]. 临床耳鼻咽喉头颈外科杂志, 2022, 36(11): 888-892. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2022.11.017
[8] 张秩荻, 马芙蓉, 刘俊秀, 等. 鼻分泌物Ⅱ型炎症细胞因子在嗜酸粒细胞型慢性鼻窦炎伴鼻息肉中的表达及其预测价值[J]. 临床耳鼻咽喉头颈外科杂志, 2022, 36(12): 934-939. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2022.12.009
[9] de Leval L, Jaffe ES. Lymphoma Classification[J]. Cancer J, 2020, 26(3): 176-185. doi: 10.1097/PPO.0000000000000451
[10] 李小秋, 李甘地, 高子芬, 等. 中国淋巴瘤亚型分布: 国内多中心性病例10002例分析[J]. 诊断学理论与实践, 2012, 11(2): 111-115. https://www.cnki.com.cn/Article/CJFDTOTAL-ZDLS201202007.htm
[11] Peng KA, Kita AE, Suh JD, et al. Sinonasal lymphoma: case series and review of the literature[J]. Int Forum Allergy Rhinol, 2014, 4(8): 670-674. doi: 10.1002/alr.21337
[12] 杨双双, 巴云鹏, 姜国忠. CD20阳性的结外鼻型NK/T细胞淋巴瘤11例临床分析[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(5): 436-440. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2021.05.012
[13] 王斐璇, 卢曲琴, 邵立健. 鼻型NK/T细胞淋巴瘤研究进展[J]. 国际耳鼻咽喉头颈外科杂志, 2022, 46(3): 158-161. https://www.cnki.com.cn/Article/CJFDTOTAL-YXZS201604025.htm
[14] Varelas AN, Ganti A, Eggerstedt M, et al. Prognostic indicators of survival in sinonasal extranodal natural killer/T-cell lymphoma[J]. Laryngoscope, 2019, 129(12): 2675-2680.
[15] Brown HJ, Varelas EA, Ganti A, et al. Prognostic Indicators of Survival in Sinonasal Diffuse Large B-Cell Lymphoma: A National Cancer Database Analysis[J]. Laryngoscope, 2022, 132(8): 1515-1522.
[16] 孔雪, 董昭, 刘海涛, 等. 基于16S rDNA高通量测序分析老年睡眠障碍患者肠道菌群特征[J]. 空军军医大学学报, 2022, 43(7): 729-733. https://www.cnki.com.cn/Article/CJFDTOTAL-DSJY202207014.htm
[17] Kumpitsch C, Koskinen K, Schopf V, et al. The microbiome of the upper respiratory tract in health and disease[J]. BMC Biol, 2019, 17(1): 87.
[18] Thangaleela S, Sivamaruthi BS, Kesika P, et al. Nasal Microbiota, Olfactory Health, Neurological Disorders and Aging-A Review[J]. Microorganisms, 2022, 10(7): 1405.
[19] Proctor DM, Relman DA. The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat[J]. Cell Host Microbe, 2017, 21(4): 421-432. http://www.onacademic.com/detail/journal_1000039877398910_cdb3.html
[20] Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus[J]. Virulence, 2021, 12(1): 547-569.
[21] Kwiecinski JM, Horswill AR. Staphylococcus aureus bloodstream infections: athogenesis and regulatory mechanisms[J]. Curr Opin Microbiol, 2020, 53: 51-60.
[22] Laux C, Peschel A, Krismer B. Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members[J]. Microbiol Spectr, 2019, 7(2). doi: 10.1128/microbiolspec.GPP3-0029-2018.
[23] Li L, Yu R, Cai T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment[J]. Int Immunopharmacol, 2020, 88: 106939.
[24] Zhang Z, Hu Y, Chen Y, et al. Immunometabolism in the tumor microenvironment and its related research progress[J]. Front Oncol, 2022, 12: 1024789.
[25] Cook MR, Dunleavy K. Targeting The Tumor Microenvironment in Lymphomas: Emerging Biological Insights and Therapeutic Strategies[J]. Curr Oncol Rep, 2022, 24(9): 1121-1131.
[26] 卞真真, 聂山林, 桑威, 等. 淋巴瘤微环境与免疫逃逸关系的研究进展[J]. 白血病·淋巴瘤, 2021, 30(4): 253-256.
[27] Ciernikova S, Sevcikova A, Stevurkova V, et al. Tumor microbiome-an integral part of the tumor microenvironment[J]. Front Oncol, 2022, 12: 1063100.
-

| 引用本文: | 徐冰清, 于龙刚, 姜彦, 等. 鼻部淋巴瘤患者鼻分泌物的细菌学分析[J]. 临床耳鼻咽喉头颈外科杂志, 2023, 37(4): 247-251. doi: 10.13201/j.issn.2096-7993.2023.04.002 |
| Citation: | XU Bingqing, YU Longgang, JIANG Yan, et al. Bacteriological analysis of nasal secretions in patients with nasal lymphoma[J]. J Clin Otorhinolaryngol Head Neck Surg, 2023, 37(4): 247-251. doi: 10.13201/j.issn.2096-7993.2023.04.002 |
- Figure 1.



 下载:
下载: