Efficacy and safety assessment of transnasal nebulisation of budesonide in children with adenoid hypertrophy
-
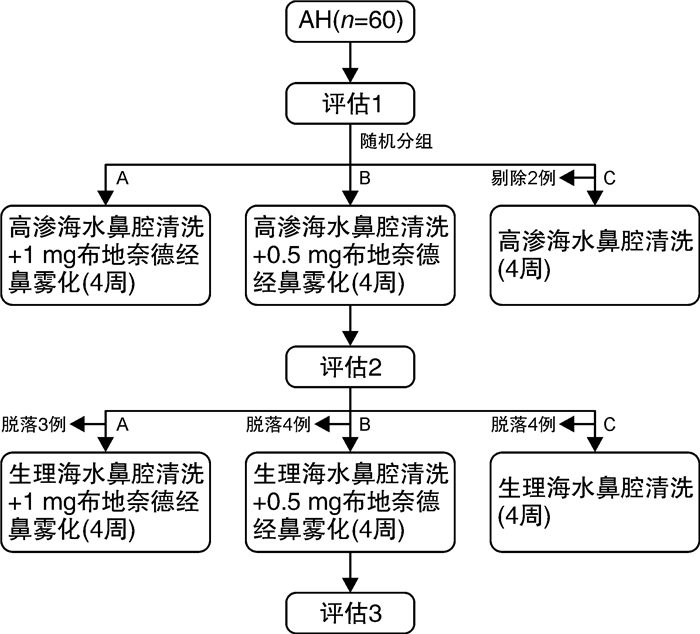
摘要: 目的 探究布地奈德经鼻雾化对儿童腺样体肥大的疗效及并对其安全性进行研究。方法 将2021年12月-2022年12月在浙江大学医学院附属儿童医院就诊的腺样体肥大患儿,随机分配至布地奈德高剂量组(A组:布地奈德1 mg/次+盐水鼻腔冲洗)、布地奈德低剂量组(B组:布地奈德0.5 mg/次+盐水鼻腔冲洗)、对照组(C组:盐水鼻腔冲洗),每组收集20例儿童,比较3组在8周治疗期间症状VAS评分、腺样体鼻咽侧位片A/N值、夜间睡眠血氧饱和度(SaO2)及不良事件发生情况,评估布地奈德经鼻雾化对儿童腺样体肥大的疗效及安全性。结果 腺样体A/N值的8周基线差值A组与B组(P < 0.001)、A组与C组(P=0.022)组间比较差异有统计学意义,A组腺样体体积前后改变量最为明显,与其他2组存在差异。随着干预时间的增加,3组SaO2水平逐渐上升(F=154.725,P < 0.001),VAS评分逐渐下降(F=165.616,P < 0.001)。治疗8周后,A组的SaO2及VAS总分的改善程度较B组高;A组与B组、A组与C组SaO2水平及VAS总分比较差异均有统计学意义(P < 0.01);3组经干预后,A组的治疗方案对VAS总分和SaO2的改善程度最大,其次是B组。在整个试验过程中,3组不良事件发生率差异无统计学意义。结论 布地奈德混悬液经鼻雾化对腺样体肥大患儿的治疗具有良好的有效性和安全性,有利于缩小腺样体体积,改善腺样体肥大患儿的临床症状,提高夜间睡眠SaO2,具有一定的临床应用价值。Abstract: Objective To investigate the efficacy and assess the safety of transnasal nebulisation of budesonide in children with adenoid hypertrophy.Methods Children with adenoid hypertrophy who attended the Children's Hospital of Zhejiang University School of Medicine between December 2021 and December 2022 were randomly assigned to budesonide high-dose group(Group A: budesonide 1 mg/dose + saline nasal rinse), budesonide low-dose group(Group B: budesonide 0.5 mg/dose + saline nasal rinse), and control group(Group C: saline nasal rinse), and each group 20 children were collected separately, The efficacy and safety of transnasal nebulisation of budesonide in children with adenoid hypertrophy were assessed by comparing the symptomatic VAS scores, adenoidal nasopharyngeal lateral radiographs A/N values, nocturnal sleep oximetry(SaO2), and the incidence of adverse events during the treatment period of 8-week in the three groups.Results The 8-week baseline differences in adenoid A/N values were statistically different between groups A and B(P < 0.001) and A and C(P=0.022), with the reduced amount in adenoid volume being most pronounced in group A, which differed from the other two groups. With the increase of intervention time, SaO2 levels gradually increased(F=154.725, P < 0.001) and VAS scores gradually decreased(F=165.616, P < 0.001) in all three groups. After 8 weeks of treatment, there was no statistically significant difference in SaO2 level(P=0.085) between groups A and B. There was a statistically significant difference in VAS total scores between Group A and Group B (P < 0.05). The improvement of SaO2and total VAS score in group A was higher than that in group B. There was a statistically significant difference in the comparison of SaO2 level and total VAS score between groups A and B, and between groups A and C(P < 0.01); after the intervention of the three groups, showing the greatest improvement of total VAS score and SaO2in the group A, followed by Group B. There was no statistically significant difference in the incidence of adverse events among Groups A, B, and C throughout the trial.Conclusion The treatment of children with adenoid hypertrophy by intranasal nebulisation of budesonide suspension has good efficacy and safety, which is conducive to reducing the size of adenoids, improving the clinical symptoms of children with adenoid hypertrophy, and improving the SaO2of nocturnal sleep, and it has a certain clinical application value.
-
Key words:
- Budesonide /
- nebulization inhalation /
- children /
- adenoid hypertrophy /
- drug clinical trials
-

-
表 1 各组不同疗程的治疗方案
组别 第1疗程(28 d) 第2疗程(28 d) A组 高渗海水鼻腔清洗液鼻腔冲洗,7 mL/鼻/次(7 mL/支,2支/次) 洗鼻结束10 min后,布地奈德混悬液经鼻雾化吸入1 mg/次(配3 mL生理盐水稀释用) 早晚各1次 生理盐水鼻腔清洗液鼻腔冲洗,7 mL/鼻/次(7 mL/支,2支) 洗鼻结束10 min后,布地奈德混悬液经鼻雾化吸入1 mg/次(配3 mL生理盐水稀释用) 早晚各1次 B组 高渗海水鼻腔清洗液鼻腔冲洗,7 mL/鼻/次(7 mL/支,2支) 洗鼻结束10 min后,布地奈德混悬液经鼻雾化吸入0.5 mg/次(半支/次,配3 mL生理盐水稀释用) 早晚各1次 生理盐水鼻腔清洗液鼻腔冲洗,7 mL/鼻/次(7 mL/支,2支)洗鼻结束10 min后,布地奈德混悬液经鼻雾化吸入0.5 mg/次(半支/次,配3 mL生理盐水稀释用) 早晚各1次 C组 高渗海水鼻腔清洗液鼻腔冲洗,7 mL/鼻/次(7 mL/支,2支) 早晚各1次 生理盐水鼻腔清洗液鼻腔冲洗,7 mL/鼻/次(7 mL/支,2支) 早晚各1次 表 2 3组基线数据的比较
X±S 指标 A组(n=20) B组(n=20) C组(n=18) F P 身高/cm 117.68±7.63 117.72±5.65 118.17±9.26 0.024 0.976 体重/kg 22.54±5.07 22.30±3.67 24.07±6.07 0.693 0.504 A/N比值 0.71±0.06 0.70±0.06 0.71±0.06 0.186 0.831 VAS评分 鼻塞 6.25±1.97 6.00±1.65 6.06±2.46 0.052 0.950 打鼾 4.85±1.81 4.50±1.70 4.83±1.50 0.179 0.836 咳嗽 3.15±1.87 3.20±1.88 3.94±1.66 1.122 0.333 张口呼吸 6.45±2.11 6.60±1.39 6.39±2.06 0.078 0.925 总分 20.70±5.48 20.30±4.10 21.22±4.19 0.224 0.800 血氧 91.60±1.54 92.00±1.30 91.50±1.15 0.773 0.467 脉搏 87.60±5.32 87.45±9.00 87.56±6.11 0.002 0.998 表 3 3组治疗前后腺样体A/N值比较
X±S 组别 例数 治疗前 治疗4周后 治疗8周后 F P A组 20 0.71±0.065 0.68±0.08 0.63±0.1182) 4.439 0.016 B组 20 0.70±0.056 0.69±0.08 0.72±0.0881) 0.835 0.439 C组 18 0.71±0.056 0.67±0.08 0.69±0.0921) 1.129 0.331 F 0.186 0.194 4.239 P 0.831 0.824 0.019 与A组比较,1)P < 0.05;与干预前比较,2)P < 0.05。 表 4 3组治疗前后腺样体A/N值基线差值比较
指标 A组(n=20) B组(n=20) C组(n=18) F/χ2 P A/N比值 随访4周-基线 -0.03±0.05 -0.01±0.06 -0.04±0.07 1.012 0.370 随访8周-基线 -0.08±0.09 0.02±0.06 -0.02±0.08 7.981 0.001 表 5 3组睡眠血氧及VAS总分的重复测量方差分析结果
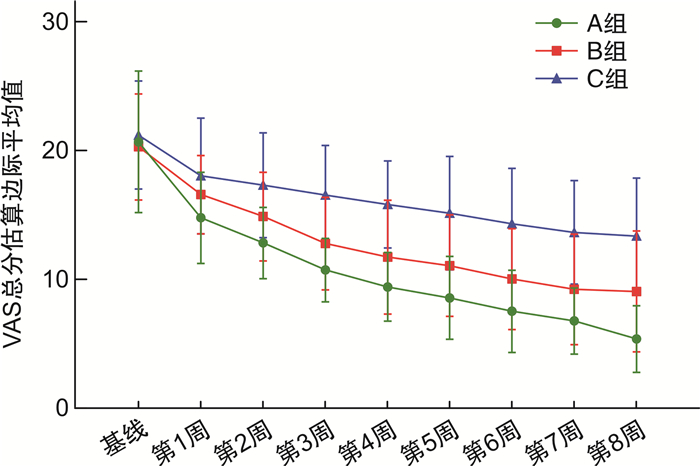
X±S 指标 VAS总分 血氧评分 A组(n=20) B组(n=20) C组(n=18) A组(n=20) B组(n=20) C组(n=18) 基线 20.70±5.48 20.30±4.10 21.22±4.19 91.60±1.54 92.00±1.30 91.50±1.15 t1 14.80±3.55 16.60±3.03 18.06±4.49 92.85±1.60 92.40±1.85 92.06±1.39 t2 12.85±2.76 14.90±3.45 17.33±4.06 93.20±1.96 93.35±2.08 92.50±1.54 t3 10.75±2.47 12.80±3.59 16.56±3.85 95.10±1.89 94.10±2.10 92.72±1.56 t4 9.45±2.67 11.75±4.41 15.83±3.38 96.10±2.02 93.80±2.04 92.56±1.58 t5 8.60±3.20 11.10±3.93 15.17±4.40 96.30±2.05 95.15±1.93 92.89±2.11 t6 7.55±3.19 10.05±3.91 14.33±4.30 96.65±1.76 95.90±1.71 93.44±1.98 t7 6.80±2.57 9.25±4.28 13.67±4.01 97.20±1.79 96.70±1.52 93.44±2.23 t8 5.40±2.58 9.10±4.68 13.39±4.49 97.70±1.81 96.75±1.25 93.44±2.38 F F干预效应=16.594 F时间效应=165.616 F交互效应=4.991 F干预效应=17.356 F时间效应=154.725 F交互效应=12.831 P P干预效应 < 0.001 P时间效应 < 0.001 P交互效应=0.010 P干预效应 < 0.001 P时间效应 < 0.001 P交互效应 < 0.001 表 6 不良事件发生情况(SS)
不良事件 A组
(n=20)B组
(n=20)C组
(n=20)例数(%) 感冒 2 4 2 8(13.30) 咳嗽 1 2 2 5(8.33) 鼻塞 0 0 1 1(1.67) 流涕 0 1 2 3(5.00) 打喷嚏 1 0 0 1(1.67) 喉炎 1 0 1 2(3.33) 流鼻血 1 0 2 3(5.00) 中耳炎 0 0 1 1(1.67) 眼部过敏 0 1 0 1(1.67) 发烧 2 1 1 4(6.67) 炎症 1 0 0 1(1.67) 假性发育 1 0 0 1(1.67) -
[1] Pereira L, Monyror J, Almeida FT, et al. Prevalence of adenoid hypertrophy: A systematic review and meta-analysis[J]. Sleep Med Rev, 2018, 38: 101-112. doi: 10.1016/j.smrv.2017.06.001
[2] 中国儿童OSA诊断与治疗指南制订工作组, 中华医学会耳鼻咽喉头颈外科学分会小儿学组, 中华医学会儿科学分会呼吸学组, 等. 中国儿童阻塞性睡眠呼吸暂停诊断与治疗指南(2020)[J]. 中国循证医学杂志, 2020, 20(8): 883-900.
[3] 深圳市医师协会等离子医疗专业委员会. 低温等离子射频消融术治疗儿童扁桃体、腺样体肥大操作技术规范[J]. 临床耳鼻咽喉头颈外科杂志, 2023, 37(8): 593-604. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2023.08.001
[4] 中华医学会呼吸病学分会哮喘学组. 上-下气道慢性炎症性疾病联合诊疗与管理专家共识[J]. 中华医学杂志, 2017, 97(26): 2001-2022. doi: 10.3760/cma.j.issn.0376-2491.2017.26.001
[5] 上海市医学会儿科分会呼吸学组, 江苏省医学会儿科分会呼吸学组, 福建省医学会儿科分会呼吸学组, 等. 鼻腔盐水冲洗在儿童上呼吸道感染的应用专家共识[J]. 中华实用儿科临床杂志, 2023, 38(7): 486-490. doi: 10.3760/cma.j.cn101070-20230120-00055
[6] Kheirandish-Gozal L, Serpero LD, Dayyat E, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea[J]. Eur Respir J, 2009, 33(5): 1077-1084. doi: 10.1183/09031936.00130608
[7] Goldbart AD, Veling MC, Goldman JL, et al. Glucocorticoid receptor subunit expression in adenotonsillar tissue of children with obstructive sleep apnea[J]. Pediatr Res, 2005, 57(2): 232-236. doi: 10.1203/01.PDR.0000150722.34561.E6
[8] Rezende RM, Silveira F, Barbosa AP, et al. Objective reduction in adenoid tissue after mometasone furoate treatment[J]. Int J Pediatr Otorhinolaryngol, 2012, 76(6): 829-831. doi: 10.1016/j.ijporl.2012.02.052
[9] Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome[J]. Pediatrics, 2008, 122(1): e149-155. doi: 10.1542/peds.2007-3398
[10] Shah SA, Berger RL, McDermott J, et al. Regional deposition of mometasone furoate nasal spray suspension in humans[J]. Allergy Asthma Proc, 2015, 36(1): 48-57. doi: 10.2500/aap.2015.36.3817
[11] 中华医学会呼吸病学分会《雾化吸入疗法在呼吸疾病中的应用专家共识》制定专家组. 雾化吸入疗法在呼吸疾病中的应用专家共识[J]. 中华医学杂志, 2016, 96(34): 2696-2708. doi: 10.3760/cma.j.issn.0376-2491.2016.34.003
[12] Cai Y, Gudis DA. Is topical high-volume budesonide sinus irrigation safe?[J]. Laryngoscope, 2018, 128(4): 781-782. doi: 10.1002/lary.26880
[13] Bourhis T, Mouawad F, Szymanski C, et al. Budesonide transnasal pulsating nebulization after surgery in chronic rhinosinusitis with nasal polyps[J]. Drug Deliv Transl Res, 2022, 12(4): 925-930. doi: 10.1007/s13346-021-00979-6
[14] Hong H, Chen F, Zheng X, et al. Decreased frequency of adenoidectomy by a 12-week nasal budesonide treatment[J]. Ther Clin Risk Manag, 2017, 13: 1309-1316. doi: 10.2147/TCRM.S144659
[15] 陈文欣, 付勇. 腺样体肥大在儿童分泌性中耳炎发病机制中的作用研究进展[J]. 临床耳鼻咽喉头颈外科杂志, 2018, 32(17): 1359-1362. doi: 10.13201/j.issn.1001-1781.2018.17.017
[16] Ramadan HH, Chaiban R, Makary C. Pediatric Rhinosinusitis[J]. Pediatr Clin North Am, 2022, 69(2): 275-286. doi: 10.1016/j.pcl.2022.01.002
[17] 中华耳鼻咽喉头颈外科杂志编辑委员会鼻科组, 中华医学会耳鼻咽喉头颈外科学分会鼻科学组. 中国慢性鼻窦炎诊断和治疗指南(2018)[J]. 中华耳鼻咽喉头颈外科杂志, 2019, 54(2): 81-100. doi: 10.3760/cma.j.issn.1673-0860.2019.02.001
[18] 张云飞, 黄钺, 段博, 等. 不同剂量布地奈德混悬液雾化吸入联合盐水鼻腔冲洗治疗腺样体肥大患儿的随机对照研究[J]. 中国实用儿科杂志, 2023, 38(5): 368-375.
[19] Wang C, Lou H, Wang X, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps[J]. J Allergy Clin Immunol, 2015, 135(4): 922-929. e6. doi: 10.1016/j.jaci.2014.10.018
[20] Zhang M, Ni JZ, Cheng L. Safety of intranasal corticosteroids for allergic rhinitis in children[J]. Expert Opin Drug Saf, 2022, 21(7): 931-938. doi: 10.1080/14740338.2022.2046731
[21] Schenkel EJ, Skoner DP, Bronsky EA, et al. Absence of growth retardation in children with perennial allergic rhinitis after one year of treatment with mometasone furoate aqueous nasal spray[J]. Pediatrics, 2000, 105(2): E22. doi: 10.1542/peds.105.2.e22
-




 下载:
下载: