Adipose-derived stem cell-derived exosomes regulate Th2/Treg balance in peripheral blood of AR patients through the mTOR pathway
-
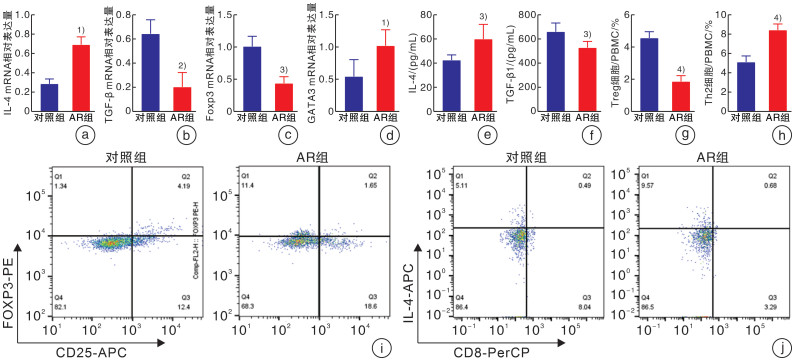
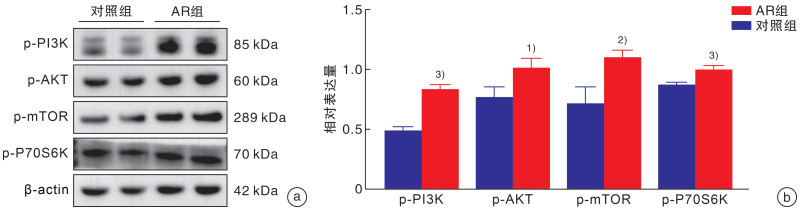
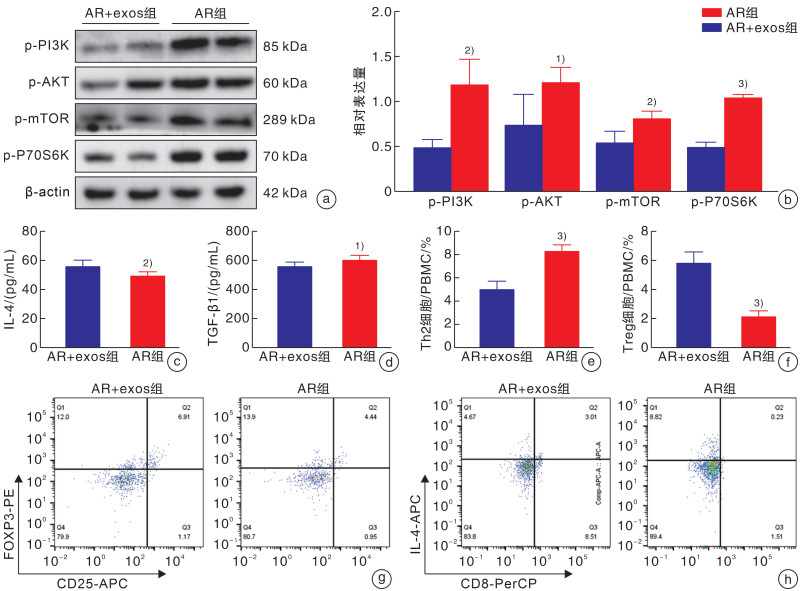
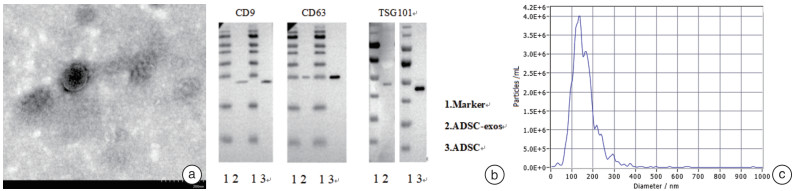
摘要: 目的 探讨脂肪间充质干细胞来源外泌体(adipose-derived stem cell exosomes,ADSC-exos)调节变应性鼻炎(allergic rhinitis,AR)患者外周血Th2/Treg平衡的机制。 方法 选取2022年3月-2022年10月于郑州大学第一附属医院耳鼻咽喉头颈外科就诊的AR患者30例,再选择30例同期就诊的单纯鼻中隔偏曲患者作为对照组。期间收集所有患者的外周静脉血10 mL,采用ELISA检测法分析血浆中IL-4、TGF-β细胞因子水平,密度梯度离心法提取外周血单个核细胞(peripheral blood mononuclear cells,PBMCs)后进一步提取蛋白及RNA,行qRT-PCR检测IL-4、TGF-β、GATA3、Foxp3基因的表达水平,Western Blotting检测AR患者与对照者PBMCs中p-PI3K(P85)、p-AKT(Ser473)、p-mTOR(Ser2448)、p-p70S6K(Thr389)的蛋白表达水平,流式细胞术分析Th2和Treg细胞的比例。刺激分化AR患者的PBMCs,并与ADSC-exos共培养。Western Blotting检测外泌体处理组与未处理组p-PI3K(P85)、p-AKT(Ser473)、p-mTOR(Ser2448)及p-p70S6K(Thr389)蛋白的表达水平,流式细胞术分析Th2和Treg细胞的比例,ELISA检测细胞培养上清中IL-4、TGF-β的水平。 结果 AR组患者外周血中mTOR通路较对照组显著激活,血浆中IL-4水平较对照组升高,TGF-β水平较对照组降低,差异均有统计学意义(P<0.05)。AR组患者外周血Th2细胞比例较对照组升高,Treg细胞比例下降,差异有统计学意义(P<0.01)。外泌体处理组与未处理组比较,mTOR通路蛋白表达水平下降,IL-4水平下降,TGF-β水平升高; Th2细胞比例下降,Treg细胞比例升高(P<0.01)。 结论 AR患者外周血单个核细胞中存在Th2、Treg细胞的失衡,其外周血单个核细胞中PI3K/AKT/mTOR/p70S6K通路被激活,ADSC-exos可能通过PI3K/AKT/mTOR/p70S6K通路调节AR患者Th2/Treg的平衡。Abstract: Objective To investigate the mechanism of adipose derived stem cell exosomes(ADSC-exos) regulating Th2/Treg balance in peripheral blood of patients with allergic rhinitis(AR). Methods Thirty patients with AR who were treated in Department of Otolaryngology Head and Neck Surgery, the First Affiliated Hospital of Zhengzhou University from March 2022 to October 2022 were selected, and 30 patients with simple deviation of nasal septum who were treated in our department during the same period were selected as the control group. 10 mL peripheral venous blood was collected from all patients. The levels of IL-4 and TGF-β in plasma were analyzed by ELISA. PBMCs were isolated by density gradient centrifugation. Then, protein and RNA were further extracted, and the expression levels of IL-4, TGF-β, GATA3 and Foxp3 genes were detected by qRT-PCR. Western Blotting detected p-PI3K(P85), p-AKT(Ser473) in PBMCs of AR patients and healthy controls. Protein expression levels of p-mTOR(Ser2448), p-p70S6K(Thr389), and the proportion of Th2 and Treg cells were analyzed by flow cytometry. PBMCs of AR patients were stimulated to differentiate and co-cultured with exosomes of adipose stem cells. p-PI3K(P85), p-AKT(Ser473), p-mTOR(Ser2448) were detected in exosome treated group and untreated group by Western Blotting. The expression level of p-p70S6K(Thr389) protein, the proportion of Th2 and Treg cells were analyzed by flow cytometry, and the levels of IL-4 and TGF-β in the supernatant of cell culture were detected by ELISA. Results Compared with the control group, the mTOR pathway in peripheral blood of AR group was significantly activated, the level of IL-4 in plasma was increased, and the level of TGF-β was decreased(P < 0.05). Compared with the control group, the proportion of Th2 cells in peripheral blood was increased, and the proportion of Treg cells was decreased(P < 0.01). Compared with the untreated group, the expression level of mTOR pathway protein decreased, the level of IL-4 decreased, and the level of TGF-β increased. The proportion of Th2 cells decreased, and the proportion of Treg cells increased(P < 0.01). Conclusion There is an imbalance of Th2 and Treg cells in peripheral blood mononuclear cells of AR patients; the PI3K/AKT/mTOR/p70S6K pathway is activated in peripheral blood mononuclear cells of AR patients Exosomes derived from adipose mesenchymal stem cells may regulate Th2/Treg balance in AR patients through the PI3K/AKT/mTOR/p70S6K pathway.
-
Key words:
- adipose derived stem cell /
- exosomes /
- mTOR pathway /
- Th2 cell /
- Treg cell
-

-
[1] Fan Y, Piao CH, Hyeon E, et al. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis[J]. Int Immunopharmacol, 2019, 70: 512-519. doi: 10.1016/j.intimp.2019.02.025
[2] Wang YH, Liu YJ. The IL-17 cytokine family and their role in allergic inflammation[J]. Curr Opin Immunol, 2008, 20(6): 697-702. doi: 10.1016/j.coi.2008.09.004
[3] Girtsman T, Jaffar Z, Ferrini M, et al. Natural Foxp3(+) regulatory T cells inhibit Th2 polarization but are biased toward suppression of Th17-driven lung inflammation[J]. J Leukoc Biol, 2010, 88(3): 537-546. doi: 10.1189/jlb.0110044
[4] Roy A, Srivastava M, Saqib U, et al. Potential therapeutic targets for inflammation in toll-like receptor 4(TLR4)-mediated signaling pathways[J]. Int Immunopharmacol, 2016, 40: 79-89. doi: 10.1016/j.intimp.2016.08.026
[5] Gao S, Zhang W, Zhao Q, et al. Curcumin ameliorates atherosclerosis in apolipoprotein E deficient asthmatic mice by regulating the balance of Th2/Treg cells[J]. Phytomedicine, 2019, 52: 129-135. doi: 10.1016/j.phymed.2018.09.194
[6] Shao Y, Chong L, Lin P, et al. MicroRNA-133a alleviates airway remodeling in asthtama through PI3K/AKT/mTOR signaling pathway by targeting IGF1R[J]. J Cell Physiol, 2019, 234(4): 4068-4080. doi: 10.1002/jcp.27201
[7] Zou W, Ding F, Niu C, et al. Brg1 aggravates airway inflammation in asthma via inhibition of the PI3K/Akt/mTOR pathway[J]. Biochem Biophys Res Commun, 2018, 503(4): 3212-3218. doi: 10.1016/j.bbrc.2018.08.127
[8] Fan XL, Zeng QX, Li X, et al. Induced pluripotent stem cell-derived mesenchymal stem cells activate quiescent T cells and elevate regulatory T cell response via NF-κB in allergic rhinitis patients[J]. Stem Cell Res Ther, 2018, 9(1): 170. doi: 10.1186/s13287-018-0896-z
[9] Cho KS, Park HK, Park HY, et al. IFATS collection: Immunomodulatory effects of adipose tissue-derived stem cells in an allergic rhinitis mouse model[J]. Stem Cells, 2009, 27(1): 259-265. doi: 10.1634/stemcells.2008-0283
[10] Du YM, Zhuansun YX, Chen R, et al. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma[J]. Exp Cell Res, 2018, 363(1): 114-120. doi: 10.1016/j.yexcr.2017.12.021
[11] 潘志宇, 余少卿. 外泌体在变应性鼻炎发病机制中的研究进展[J]. 国际耳鼻咽喉头颈外科杂志, 2022, 46(2): 92-95.
[12] Zhang Y, Zhang L. Prevalence of allergic rhinitis in china[J]. Allergy Asthma Immunol Res, 2014, 6(2): 105-113. doi: 10.4168/aair.2014.6.2.105
[13] 张珒珒, 崔晏文, 高亚东. 变应性鼻炎合并气道高反应性的风险因素研究进展[J]. 临床耳鼻咽喉头颈外科杂志, 2023, 37(6): 457-462. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2023.06.010
[14] Guo L, Huang Y, Chen X, et al. Innate immunological function of TH2 cells in vivo[J]. Nature Immunology, 2015, 16(10): 1051-1059. doi: 10.1038/ni.3244
[15] Li J, Sha J, Sun L, et al. Contribution of Regulatory T Cell Methylation Modifications to the Pathogenesis of Allergic Airway Diseases[J]. J Immunol Res, 2021, 2021: 5590217.
[16] Lin Y L, Shieh CC, Wang J Y. The functional insufficiency of human CD4+CD25 high T-regulatory cells in allergic asthma is subjected to TNF-alpha modulation[J]. Allergy, 2008, 63(1): 67-74. doi: 10.1111/j.1398-9995.2007.01526.x
[17] Li P, Tsang MS, Kan LL, et al. The Immuno-Modulatory Activities of Pentaherbs Formula on Ovalbumin-Induced Allergic Rhinitis Mice via the Activation of Th1 and Treg Cells and Inhibition of Th2 and Th17 Cells[J]. Molecules, 2021, 27(1): 239. doi: 10.3390/molecules27010239
[18] 张芳, 刘海, 张建辉. 外泌体在变应性鼻炎中的研究进展[J]. 医学研究与战创伤救治, 2023, 36(2): 209-213.
[19] Yao Y, Fan XL, Jiang D, et al. Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation[J]. Stem Cell Reports, 2018, 11(5): 1120-1135. doi: 10.1016/j.stemcr.2018.09.012
[20] Lai RC, Yeo RW, Tan KH, et al. Exosomes for drug delivery-a novel application for the mesenchymal stem cell[J]. Biotechnol Adv, 2013, 31(5): 543-551. doi: 10.1016/j.biotechadv.2012.08.008
[21] Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism[J]. Cell, 2006, 124(3): 471-484. doi: 10.1016/j.cell.2006.01.016
[22] Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing[J]. Nat Rev Mol Cell Biol, 2011, 12(1): 21-35. doi: 10.1038/nrm3025
[23] Huang S. mTOR Signaling in Metabolism and Cancer[J]. Cells, 2020, 9(10): 2278. doi: 10.3390/cells9102278
[24] Zhang Y, Jing Y, Qiao J, et al. Activation of the mTOR signaling pathway is required for asthma onset[J]. Sci Rep, 2017, 7(1): 4532. doi: 10.1038/s41598-017-04826-y
-




 下载:
下载: