Prediction model of recurrence after parathyroidectomy in secondary hyperparathyroidism
-
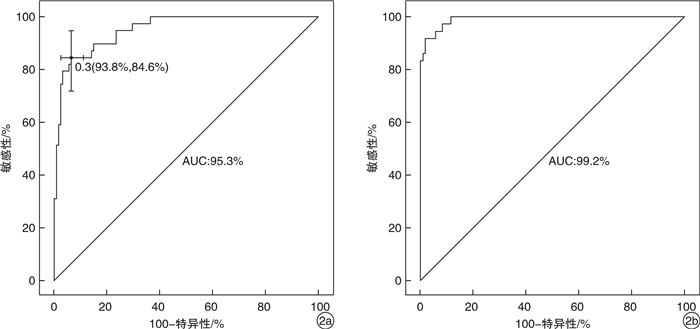
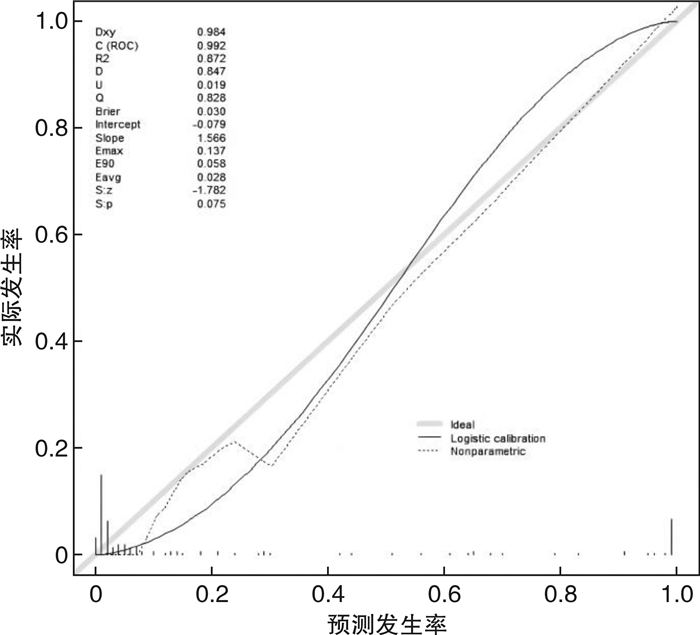
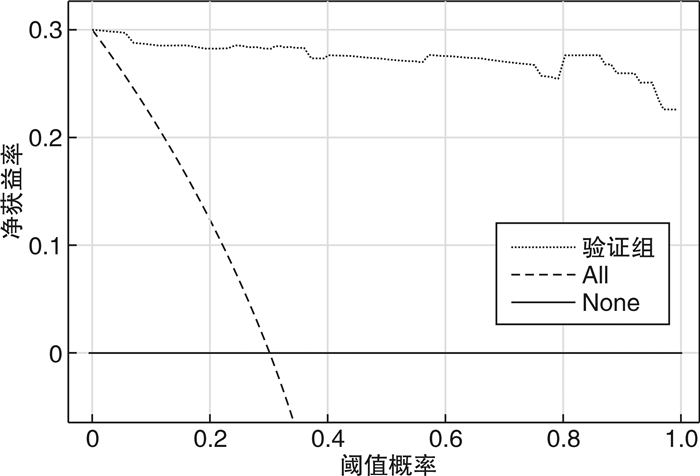
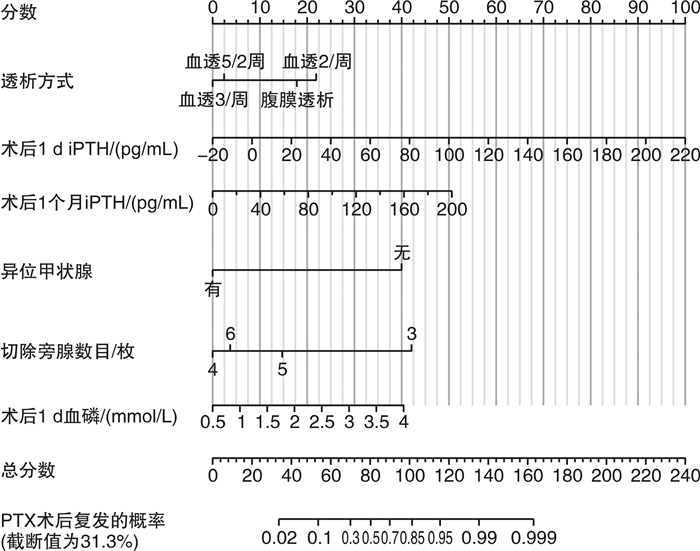
摘要: 目的 定量评估肾性继发性甲状旁腺功能亢进症(SHPT)患者接受甲状旁腺切除术(parathyroidectomy,PTX)治疗后复发的风险。方法 收集中国人民解放军北部战区总医院2017年6月—2019年5月期间接受PTX的168例患者的临床资料,通过赤池信息准则(Akaike information criterion,AIC)筛选因素、列线图的形式构建预测模型,由2019年6月—2021年9月期间接受PTX治疗的158例患者组成验证集,在区分度、一致性和临床实用性3个方面对该模型进行外部验证。结果 本研究构建的预测模型包含6个变量,分别是透析方式、异位甲状旁腺、术后1 d及术后1个月的全段甲状旁腺激素水平(iPTH)、切除甲状旁腺数目以及术后1 d的血磷,该模型外部验证的C指数为0.992,Calibration曲线的P值为0.886 1,决策曲线也显示该模型评估效果良好。结论 本研究构建的预测模型有助于判断SHPT患者行PTX术后是否复发,对其进行个体化预测。
-
关键词:
- 继发性甲状旁腺功能亢进症 /
- 甲状旁腺切除术 /
- 复发
Abstract: Objective To quantitatively evaluate the risk of recurrence in patients with secondary hyperparathyroidism after parathyroidectomy.Methods The clinical data of 168 patients who underwent parathyroidectomy(PTX) from June 2017 to May 2019 were collected. The prediction model was constructed by using Akaike information criterion(AIC) to screen factors. A total of 158 patients treated with PTX from June 2019 to September 2021 were included in the validation set to conduct external validation of the model in three aspects of differentiation, consistency and clinical utility.Results The prediction model we constructed includes different dialysis methods, ectopic parathyroid gland, the iPTH level at one day and one month after surgery, the number of excisional parathyroid and postoperative blood phosphorus. The C index of external validation of this model is 0.992 and the P value of the Calibration curve is 0.886 1. The decision curve analysis also shows that the evaluation effect of this model is perfect.Conclusion The prediction model constructed in this study is useful for individualized prediction of recurrence after PTX in patients with secondary hyperparathyroidism.-
Key words:
- secondary hyperparathyroidism /
- parathyroidectomy /
- recurrence
-

-
表 1 建模集和验证集中患者基线临床资料的比较(n=326)
因素 建模集(n=168) 验证集(n=158) 非复发组(n=129) 复发组(n=39) P值 非复发组(n=122) 复发组(n=36) P值 年龄/岁 47.6±11.1 47.1±10.2 0.805 46.9±11.0 47.1±9.4 0.933 性别/例(%) 0.100 < 0.001 女 69(53.5) 15(38.5) 51(41.8) 17(47.2) 男 60(46.5) 24(61.5) 71(58.2) 19(52.8) BMI/(kg/m2) 22.5(20.2,24.0) 22.5(20.6,23.5) 0.784 22.2(20.2,25.7) 22.3(21.0,23.6) 0.729 原发病/肾病类型/例(%) 0.580 0.271 不明原因 34(26.4) 10(25.6) 34(27.9) 15(41.7) IgA肾病 3(2.3) 1(2.6) 4(3.3) 0 高血压肾病 25(19.4) 14(35.9) 17(13.9) 6(16.7) 慢性肾小球肾炎 39(30.2) 8(20.5) 29(23.8) 8(22.2) 糖尿病 3(2.3) 1(2.6) 4(3.3) 1(2.8) 其他 25(19.4) 5(12.8) 34(27.9) 6(16.7) 透析方式/例(%) 0.042 0.467 腹膜透析 2(1.6) 2(5.1) 10(8.2) 0 血液透析(2/周) 1(0.8) 5(12.8) 6(4.9) 2(5.6) 血液透析(5/2周) 23(17.8) 6(15.4) 23(18.9) 8(22.2) 血液透析(3/周) 103(79.8) 26(66.7) 83(68.0) 26(72.2) 异位甲状旁腺/例(%) < 0.001 < 0.001 无异位甲状旁腺 121(93.8) 20(51.3) 117(95.9) 26(72.2) 有异位甲状旁腺 8(6.2) 19(48.7) 5(4.1) 10(27.8) 术前iPTH/(pg/mL) 1746.0(1368.0,2134.5) 1835.0(1250.0,2650.0) 0.272 1580.0(1277.5,1852.0) 1609.5(1105.0,2131.8) 0.751 术后10 min iPTH/(pg/mL) 311.0(179.0,483.5) 175.0(117.0,320.0) < 0.001 314.0(184.8,383.3) 353.3(205.0,420.3) 0.016 术后1 d iPTH/(pg/mL) 12.7 (7.2,19.3) 41.3 (21.7,146.0) < 0.001 9.4 (5.3,14.3) 122.9 (42.1,200.0) < 0.001 术后1个月iPTH/(pg/mL) 11.7 (5.4~33.0) 35.7 (35.7~101.7) < 0.001 8.0 (4.9,16.3) 127.5 (107.6,200.0) < 0.001 术后3个月iPTH/(pg/mL) 30.7 (10.1~50.8) 50.8 (50.8~93.0) < 0.001 78.4 (9.6,78.4) 107.0 (78.4,218.5) < 0.001 切除甲状旁腺数/例(%) < 0.001 < 0.001 6枚 1(0.8) 1(2.6) 0 1(2.8) 3枚 2(1.6) 7(17.9) 1(0.8) 4(11.1) 5枚 7(5.4) 7(17.9) 4(3.3) 14(38.9) 4枚 119(92.2) 24(61.5) 117(95.9) 17(47.2) 病理类型/例(%) 0.326 0.805 结节样增生 95(73.6) 27(69.2) 107(87.7) 31(86.1) 腺瘤样增生 34(26.4) 12(30.8) 15(12.3) 5(13.9) 术前 血钙/(mmol/L) 2.5(2.3,2.6) 2.5(2.3,2.6) 0.686 2.4±0.2 2.4±0.2 0.845 血磷/(mmol/L) 2.4±0.5 2.5±0.5 0.305 2.4(2.0,2.8) 2.6(2.2,3.1) 0.136 术后1 d 血钙/(mmol/L) 2.2±0.3 2.2±0.3 0.817 2.1(1.9,2.3) 2.2(2.0,2.3) 0.397 血磷/(mmol/L) 1.9(1.4,2.1) 2.1(1.7,2.5) < 0.001 2.0(1.6,2.2) 2.0(1.7,2.4) 0.220 出院当日 血钙/(mmol/L) 2.1±0.2 2.0±0.3 0.122 2.1±0.2 2.2±0.3 0.730 血磷/(mmol/L) 1.3±0.5 1.2±0.4 0.136 1.4±0.5 1.5±0.6 0.290 表 2 根据AIC筛选变量结果
变量 β β的95%CI OR OR的95%CI P值 下限 上限 下限 上限 透析方式 -0.054 -0.104 -0.003 0.947 0.901 0.997 0.040 异位甲状旁腺 0.381 0.262 0.499 1.464 1.300 1.647 < 0.001 术后1 d iPTH/(pg/mL) 0.003 0.002 0.004 1.003 1.002 1.004 < 0.001 术后1个月iPTH/(pg/mL) 0.002 0.001 0.003 1.002 1.001 1.003 < 0.001 切除旁腺数目 -0.108 -0.183 -0.033 0.898 0.833 0.968 0.005 术后1 d血磷/(mmol/L) 0.083 0.002 0.163 1.087 1.002 1.177 0.045 表 3 PTX术后预测复发的各个预测因子截止点
变量 敏感性 特异性 最佳约登指数 截止点 术后1 d iPTH/(pg/mL) 0.897 0.736 0.633 18.675 术后1个月iPTH/(pg/mL) 1 0.806 0.806 65.220 术后1 d血磷/(mmol/L) 0.410 0.915 0.325 2.270 -
[1] GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the global burden of disease study 2017[J]. Lancet, 2020, 395(10225): 709-733. doi: 10.1016/S0140-6736(20)30045-3
[2] Lamina C, Kronenberg F, Stenvinkel P, et al. Association of changes in bone mineral parameters with mortality in haemodialysis patients: insights from the ARO cohort[J]. Nephrol Dial Transplant, 2020, 35(3): 478-487. doi: 10.1093/ndt/gfz060
[3] 贺青卿, 田文. 慢性肾脏病继发甲状旁腺功能亢进外科临床实践中国专家共识(2021版)[J]. 中国实用外科杂志, 2021, 41(8): 841-848. doi: 10.19538/j.cjps.issn1005-2208.2021.08.01
[4] Kidney Disease: Improving Global Outcomes(Kdigo)CKD-MBD Update Work Group. KDIGO 2017 Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder(CKD-MBD)[J]. Kidney Int Suppl, 2017, 7(1): 1-59. doi: 10.1016/j.kisu.2017.04.001
[5] Steffen L, Moffa G, Müller PC, et al. Secondary hyperparathyroidism: recurrence after total parathyroidectomy with autotransplantation[J]. Swiss Med Wkly, 2019, 149: w20160.
[6] 贾晨晖, 薄少军, 王田田, 等. 继发性甲状旁腺功能亢进术后持续状态的再手术治疗[J]. 临床耳鼻咽喉头颈外科杂志, 2022, 36(11): 822-826, 834. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2022.11.003
[7] Zhu L, Cheng F, Zhu X, et al. Safety and effectiveness of reoperation for persistent or recurrent drug refractory secondary hyperparathyroidism[J]. Gland Surg, 2020, 9(2): 401-408. doi: 10.21037/gs-20-391
[8] 阚霖, 崔军, 宋永胜. 前列腺癌根治术后病理升级的预测模型[J]. 中国男科学杂志, 2022, 36(4): 36-41, 46. https://www.cnki.com.cn/Article/CJFDTOTAL-NXXX202204006.htm
[9] Gasparri G, Camandona M, Abbona GC, et al. Secondary and tertiary hyperparathyroidism: causes of recurrent disease after 446 parathyroidectomies[J]. Ann Surg, 2001, 233(1): 65-69. doi: 10.1097/00000658-200101000-00011
[10] 闻萍, 侯大卫, 曹金龙, 等. 术后血清PTH下降率预测甲状旁腺全切术后复发的临床价值[J]. 现代生物医学进展, 2017, 17(29): 5650-5653, 5764. https://www.cnki.com.cn/Article/CJFDTOTAL-SWCX201729012.htm
[11] 刘晓怡, 张喆, 谢超, 等. 不同透析方式患者行甲状旁腺切除的临床特点及术后复发情况分析[J]. 临床肾脏病杂志, 2022, 22(8): 638-644. doi: 10.3969/j.issn.1671-2390.2022.08.004
[12] Tominaga Y, Kakuta T, Yasunaga C, et al. Evaluation of parathyroidectomy for secondary and tertiary hyperparathyroidism by the parathyroid surgeons' society of Japan[J]. Ther Apher Dial, 2016, 20(1): 6-11. doi: 10.1111/1744-9987.12352
[13] Pattou FN, Pellissier LC, Noël C, et al. Supernumerary parathyroid glands: frequency and surgical significance in treatment of renal hyperparathyroidism[J]. World J Surg, 2000, 24(11): 1330-1334. doi: 10.1007/s002680010220
[14] Van Der Plas W, Kruijff S, Sidhu SB, et al. Parathyroidectomy for patients with secondary hyperparathyroidism in a changing landscape for the management of end-stage renal disease[J]. Surgery, 2021, 169(2): 275-281. doi: 10.1016/j.surg.2020.08.014
[15] Mi JP, Liao ZP, Pei XF, et al. Postsurgical evaluation of secondary nephrogenic hyperparathyroidism[J]. Curr Med Sci, 2019, 39(2): 259-264.
[16] Hiramitsu T, Tomosugi T, Okada M, et al. Intact parathyroid hormone levels localize causative glands in persistent or recurrent renal hyperparathyroidism: A retrospective cohort study[J]. PLoS One, 2021, 16(4): e0248366.
[17] Unais TM, Gangadhar P, Kolikkat N. Acute hyperparathyroid crisis: ectopic submandibular parathyroid gland the culprit[J]. Ann R Coll Surg Engl, 2021, 103(1): e7-e9.
[18] Jasim S, Kennel K. Persistent hyperparathyroidism due to ectopic parathyroid gland[J]. Endocrine, 2017, 55(1): 322-323.
[19] Uslu A, Okut G, Tercan IC, et al. Anatomical distribution and number of parathyroid glands, and parathyroid function, after total parathyroidectomy and bilateral cervical thymectomy[J]. Medicine(Baltim), 2019, 98(23): e15926.
[20] Kakuta T, Sawada K, Kanai G, et al. Parathyroid hormone-producing cells exist in adipose tissues surrounding the parathyroid glands in hemodialysis patients with secondary hyperparathyroidism[J]. Sci Rep, 2020, 10(1): 3290.
[21] Abruzzo A, Gioviale MC, Damiano G, et al. Reoperation for persistent or recurrent secondary hyperparathyroidism[J]. Acta Biomed, 2017, 88(3): 325-328.
[22] Taterra D, Wong LM, Vikse J, et al. The prevalence and anatomy of parathyroid glands: a meta-analysis with implications for parathyroid surgery[J]. Langenbecks Arch Surg, 2019, 404(1): 63-70.
[23] Evenepoel P, Meijers BK, Bammens B, et al. Phosphorus metabolism in peritoneal dialysis-and haemodialysis-treated patients[J]. Nephrol Dial Transplant, 2016, 31(9): 1508-1514.
[24] Chertow GM, Levin NW, Beck GJ, et al. In-center hemodialysis six times per week versus three times per week[J]. N Engl J Med, 2010, 363(24): 2287-2300.
-




 下载:
下载: