-
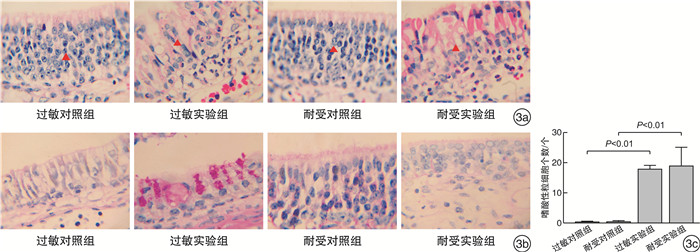
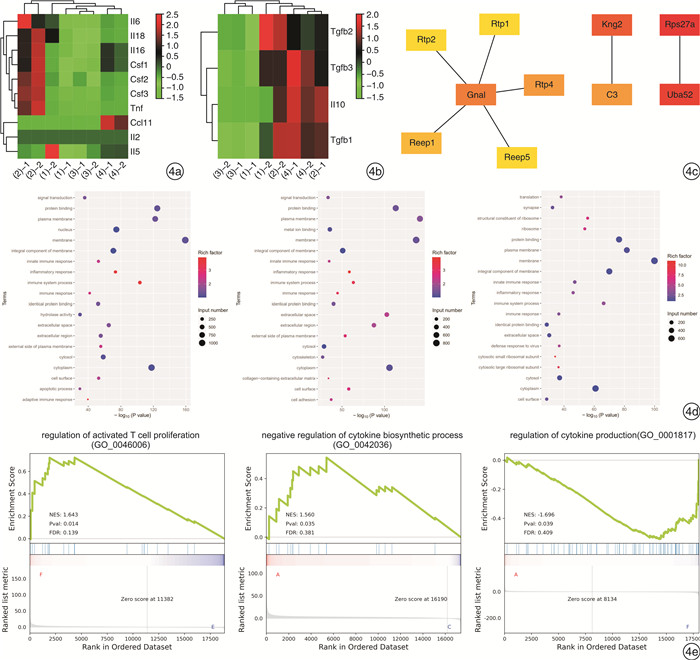
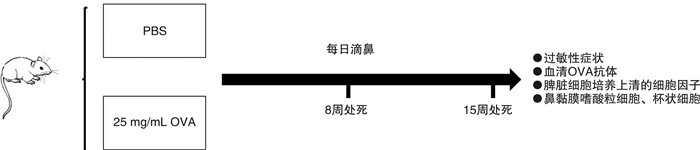
摘要: 目的 通过鼻腔滴入过敏原建立小鼠局部变应性鼻炎动物耐受模型,并对其免疫学指标及特征进行研究。方法 使用卵清蛋白(OVA)和磷酸盐缓冲液(PBS)对小鼠每日局部滴鼻,记录过敏症状,检测小鼠血清OVA特异性抗体(IgE、IgG1、IgG2a)及脾脏细胞培养上清的细胞因子(IL-4、IL-10、IFN-γ)浓度,观察鼻黏膜嗜酸粒细胞及杯状细胞的浸润情况,用RNA-seq技术分析局部鼻黏膜基因的变化。结果 小鼠在OVA刺激下首先表现为过敏症状加重,血清OVA特异性抗体IgE、IgG1、IgG2a和体外培养脾细胞上清中IL-4、IFN-γ、IL-10升高,鼻黏膜嗜酸粒细胞和杯状细胞显著升高,鼻黏膜编码IL-10、TGF-β基因表达上调,嗜酸粒细胞活化基因上调。随着滴鼻时间继续延长,过敏症状逐渐减轻,血清OVA特异性抗体IgE、IgG1、IgG2a则持续增加,体外培养脾脏细胞上清IL-4下降,IL-10升高,IFN-γ有下降的趋势,鼻黏膜杯状细胞显著减少,嗜酸粒细胞活化基因显著下调,鼻黏膜编码IL-10、TGF-β等耐受基因维持高表达。筛选出10个与局部变应性鼻炎免疫耐受相关的核心基因:Rps27a、Uba52、Kng2、Gnal、C3、Rtp4、Reep1、Rtp2、Rtp1、Reep5。结论 通过局部持续滴入过敏原,可使小鼠先形成过敏,继而产生免疫耐受。这种免疫耐受的产生,可能是在变应原的持续作用下,诱导了全身及局部的免疫耐受效应。Abstract: Objective To establish a mouse model of local allergic rhinitis tolerance by intranasal infusion of allergen, and study its immunological indexes and characteristics.Methods The mice were given ovalbumin(OVA) and phosphate buffer solution(PBS) daily, and their allergic symptoms were recorded. OVA-specific antibodies(IgE, IgG1, IgG2a) in serum and cytokine(IL-4, IL-10, IFN-γ) in splenic culture supernatant were detected. The infiltration of eosinophils and goblet cells in nasal mucosa were observed, and the changes in local nasal mucosa genes were analyzed by RNA-seq technique.Results Mice first showed aggravation of allergic symptoms when stimulated with OVA. The serum OVA-specific antibodies IgE, IgG1, IgG2a and the cytokine(IL-4, IL-10, IFN-γ) Iin splenic culture supernatant were increased, the eosinophils and goblet cells in nasal mucosa were significantly increased. The expression of encoding IL-10, TGF-β gene and eosinophils activation gene in nasal mucosa were up-regulated. As the duration of nasal dripping increased, the allergic symptoms gradually decreased, serum OVA-specific antibodies IgE, IgG1, IgG2a continued to increase. Splenic culture supernatant IL-4 decreased, IL-10 increased, IFN-γ had a downward trend. In nasal mucosa, goblet cells decreased significantly. Genes involved in eosinophils activation were significantly down-regulated. The encoding tolerance genes such as IL-10 and TGF-β genes remained highly expressed. Ten core genes associated with immune tolerance in localized allergic rhinitis were screened,Rps27a,Uba52,Kng2,Gnal,C3,Rtp4,Reep1,Rtp2,Rtp1,Reep5.Conclusion The mice first develop an allergy and then develop immune tolerance by continuous local drops of the allergen. The generation of immune tolerance may be induced by the continuous action of allergens, which induced systemic and local immune tolerance effects.
-
Key words:
- rhinitis, allergic /
- immune tolerance /
- allergen /
- mouse model
-

-
表 1 各组小鼠喷嚏数量比较
个,X±S 时间 过敏对照组 过敏实验组 耐受对照组 耐受实验组 第0周 2.33±1.80 1.33±0.84 1.83±1.34 1.50±1.26 第4周 2.17±0.9 9.25±2.862) 2.50±1.66 8.67±3.733) 第7周 2.6±1.36 14.2±3.122) 1.60±1.62 14.80±3.123) 第15周 - - 2.25±1.30 4.00±2.451) 与第7周过敏实验组比较,1)P < 0.05;与过敏对照组比较,2)P < 0.01;与耐受对照组比较,3)P < 0.01。 表 2 各组小鼠OVA特异性血清抗体水平比较
时间 过敏对照组 过敏实验组 耐受对照组 耐受实验组 IgE 第0周 0.14±0.04 0.13±0.04 0.06±0.01 0.11±0.05 第4周 0.07±0.01 0.32±0.221) 0.14±0.04 0.28±0.152) 第8周 0.03±0.00 0.55±0.301) 0.04±0.01 0.87±0.492) 第15周 - - 0.04±0.02 1.61±0.332)3) IgG1 第0周 0.01±0.00 0.01±0.00 0.01±0.00 0.01±0.00 第4周 0.01±0.00 0.30±0.221) 0.02±0.00 0.34±0.142) 第8周 0.01±0.00 0.70±0.261) 0.04±0.03 0.71±0.292) 第15周 - - 0.05±0.04 1.61±0.232)3) IgG2a 第0周 0.03±0.01 0.02±0.00 0.01±0.00 0.03±0.01 第4周 0.02±0.00 0.18±0.131) 0.02±0.00 0.36±0.092) 第8周 0.05±0.03 0.22±0.13 0.04±0.01 0.34±0.222) 第15周 - - 0.08±0.05 0.92±0.412)3) 与过敏对照组比较,1)P < 0.05;与耐受对照组比较,2)P < 0.05;与第8周过敏实验组比较,3)P < 0.05。 -
[1] Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma(ARIA)guidelines-2016 revision[J]. J Allergy Clin Immunol, 2017, 140(4): 950-958. doi: 10.1016/j.jaci.2017.03.050
[2] Wang Y, Zhou Y, Zhu Y, et al. The Comparation of Intraperitoneal Injection and Nasal-only Delivery Allergic Rhinitis Model Challenged With Different Allergen Concentration[J]. Am J Rhinol Allergy, 2019, 33(2): 145-152. doi: 10.1177/1945892418817221
[3] Durham SR, Emminger W, Kapp A, et al. SQ-standardized sublingual grass immunotherapy: confirmation of disease modification 2 years after 3 years of treatment in a randomized trial[J]. J Allergy Clin Immunol, 2012, 129(3): 717-725. e5. doi: 10.1016/j.jaci.2011.12.973
[4] Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs[J]. J Clin Invest, 2014, 124(11): 4678-4680. doi: 10.1172/JCI78891
[5] Soyer OU, Akdis M, Ring J, et al. Mechanisms of peripheral tolerance to allergens[J]. Allergy, 2013, 68(2): 161-170. doi: 10.1111/all.12085
[6] Dunston D, Ashby S, Krosnowski K, et al. An effective manual deboning method to prepare intact mouse nasal tissue with preserved anatomical organization[J]. J Vis Exp, 2013, 78: 50538.
[7] Santos MCP, Serra-Caetano A, Pedro E, et al. Expansion of FOXP3+ regulatory CD4 T cells upon exposure to hymenoptera venom during the beekeeping season[J]. Allergy, 2019, 74(6): 1182-1184. doi: 10.1111/all.13713
[8] Jutel M, Brüggenjürgen B, Richter H, et al. Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthma[J]. Allergy, 2020, 75(8): 2050-2058. doi: 10.1111/all.14240
[9] Bo ek A, Kołodziejczyk K, Kozłowska R, et al. Evidence of the efficacy and safety of house dust mite subcutaneous immunotherapy in elderly allergic rhinitis patients: a randomized, double-blind placebo-controlled trial[J]. Clin Transl Allergy, 2017, 7: 43. doi: 10.1186/s13601-017-0180-9
[10] Scadding GW, Calderon MA, Shamji MH, et al. Immune Tolerance Network GRASS Study Team. Effect of 2 Years of Treatment With Sublingual Grass Pollen Immunotherapy on Nasal Response to Allergen Challenge at 3 Years Among Patients With Moderate to Severe Seasonal Allergic Rhinitis: The GRASS Randomized Clinical Trial[J]. JAMA, 2017, 317(6): 615-625. doi: 10.1001/jama.2016.21040
[11] Maeta A, Matsushima M, Katahira R, et al. Diets Supplemented with 1% Egg White Induce Oral Desensitization and Immune Tolerance in an Egg White-Specific Allergic Mouse Model[J]. Int Arch Allergy Immunol, 2018, 176(3/4): 205-214.
[12] Gri G, Piconese S, Frossi B, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40 L interaction[J]. Immunity, 2008, 29(5): 771-781. doi: 10.1016/j.immuni.2008.08.018
[13] Zeng Q, Zeng Y, Tang Y, et al. Effect of IL-35 on apoptosis, adhesion, migration, and activation of eosinophils in allergic rhinitis[J]. Pediatr Allergy Immunol, 2022, 33(2): e13717. http://onlinelibrary.wiley.com/doi/abs/10.1111/pai.13717
[14] Lopez-Ramirez MA, Soto F, Wang C, et al. Built-In Active Microneedle Patch with Enhanced Autonomous Drug Delivery[J]. Adv Mater, 2020, 32(1): e1905740. doi: 10.1002/adma.201905740
[15] van Neerven RJ, Wikborg T, Lund G, et al. Blocking antibodies induced by specific allergy vaccination prevent the activation of CD4+ T cells by inhibiting serum-IgE-facilitated allergen presentation[J]. J Immunol, 1999, 163(5): 2944-2952.
[16] Radulovic S, Jacobson MR, Durham SR, et al. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa[J]. J Allergy Clin Immunol, 2008, 121(6): 1467-1472. doi: 10.1016/j.jaci.2008.03.013
[17] Wachholz PA, Nouri-Aria KT, Wilson DR, et al. Grass pollen immunotherapy for hayfever is associated with increases in local nasal but not peripheral Th1: Th2 cytokine ratios[J]. Immunology, 2002, 105(1): 56-62. doi: 10.1046/j.1365-2567.2002.01338.x
[18] Lilienthal GM, Rahmöller J, Petry J, et al. Potential of Murine IgG1 and Human IgG4 to Inhibit the Classical Complement and Fcγ Receptor Activation Pathways[J]. Front Immunol, 2018, 9: 958. doi: 10.3389/fimmu.2018.00958
[19] Brooks DG, Trifilo MJ, Edelmann KH, et al. Interleukin-10 determines viral clearance or persistence in vivo[J]. Nat Med, 2006, 12(11): 1301-1309. doi: 10.1038/nm1492
[20] Nouri-Aria KT, Wachholz PA, Francis JN, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity[J]. J Immunol, 2004, 172(5): 3252-3259. doi: 10.4049/jimmunol.172.5.3252
[21] Okamura T, Sumitomo S, Morita K, et al. TGF-β3-expressing CD4+CD25(-)LAG3+ regulatory T cells control humoral immune responses[J]. Nat Commun, 2015, 19;6: 6329.
[22] Fossati-Jimack L, Ling GS, Baudino L, et al. Intranasal peptide-induced tolerance and linked suppression: consequences of complement deficiency[J]. Immunology, 2015, 144(1): 149-157. doi: 10.1111/imm.12358
-

| 引用本文: | 张启迪, 祝婉婷, 邹知欣, 等. 小鼠局部变应性鼻炎耐受模型的研究[J]. 临床耳鼻咽喉头颈外科杂志, 2022, 36(12): 944-950. doi: 10.13201/j.issn.2096-7993.2022.12.011 |
| Citation: | ZHANG Qidi, ZHU Wanting, ZOU Zhixin, et al. Establishment of local allergic rhinitis tolerance in mouse model[J]. J Clin Otorhinolaryngol Head Neck Surg, 2022, 36(12): 944-950. doi: 10.13201/j.issn.2096-7993.2022.12.011 |
- Figure 1.
- Figure 2.
- Figure 3.
- Figure 4.



 下载:
下载: