Clinical significance of tumor chemosensitivity assay in patients with head and neck cancer
-
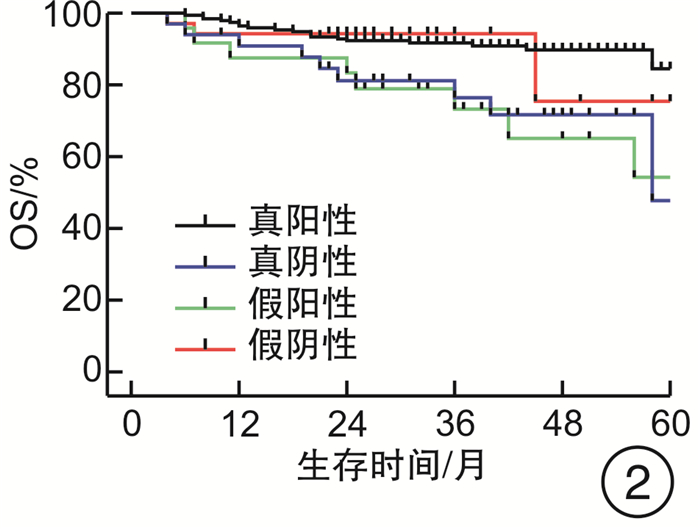
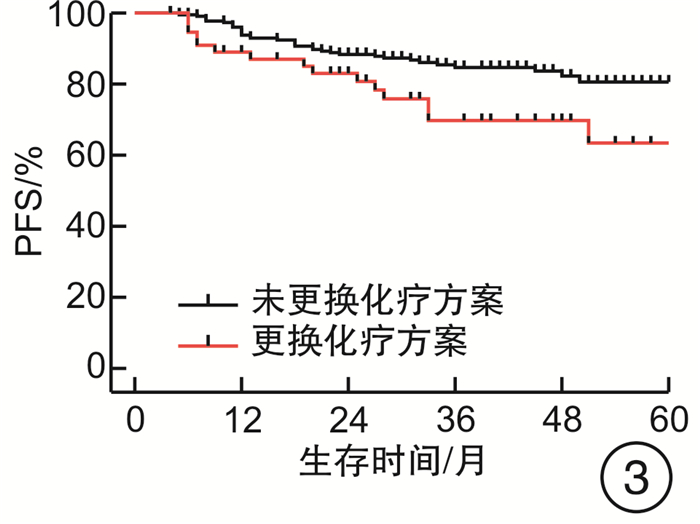
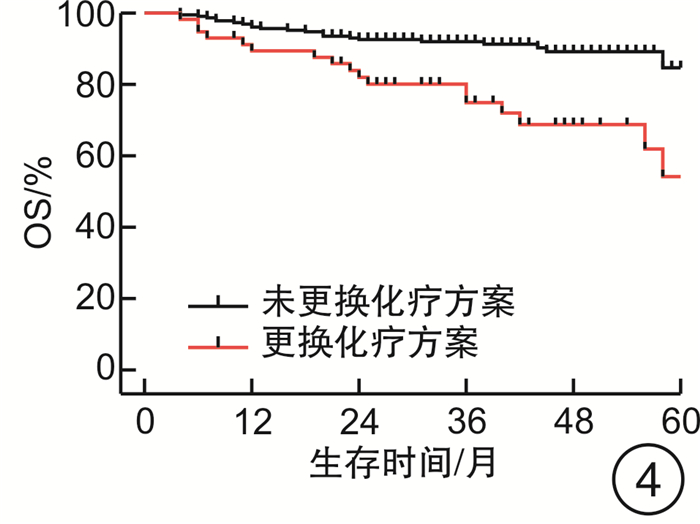
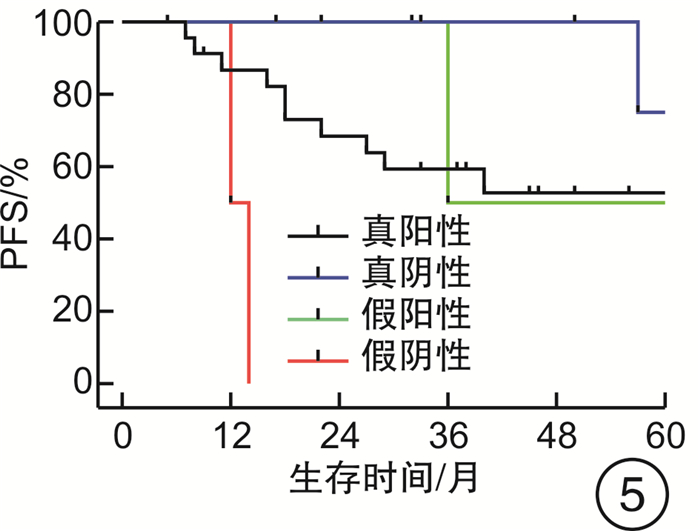
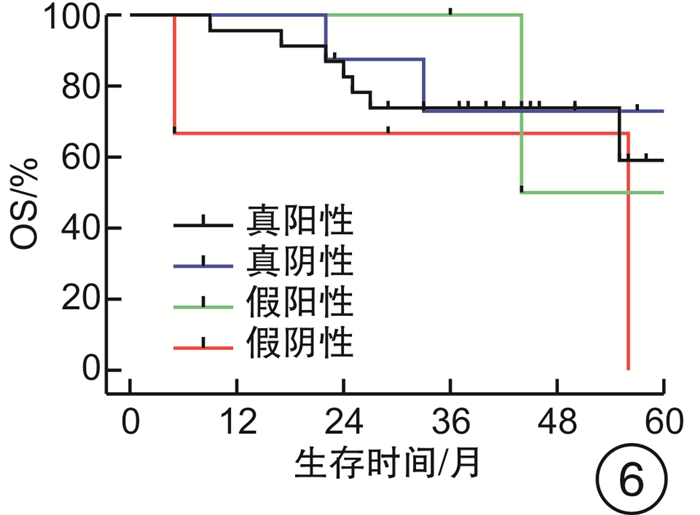
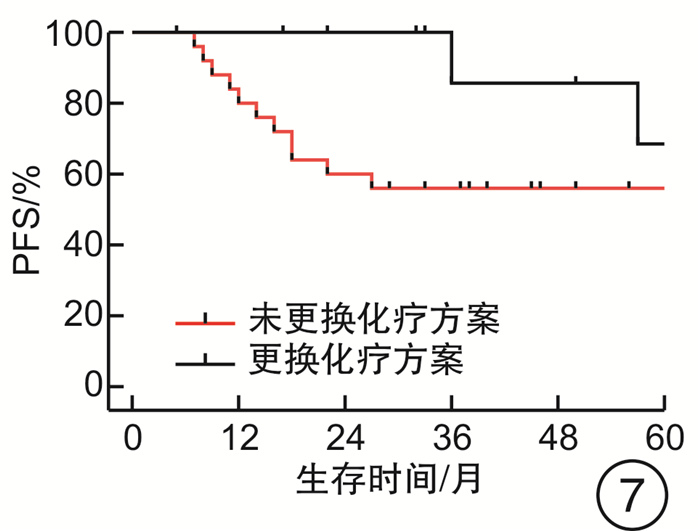
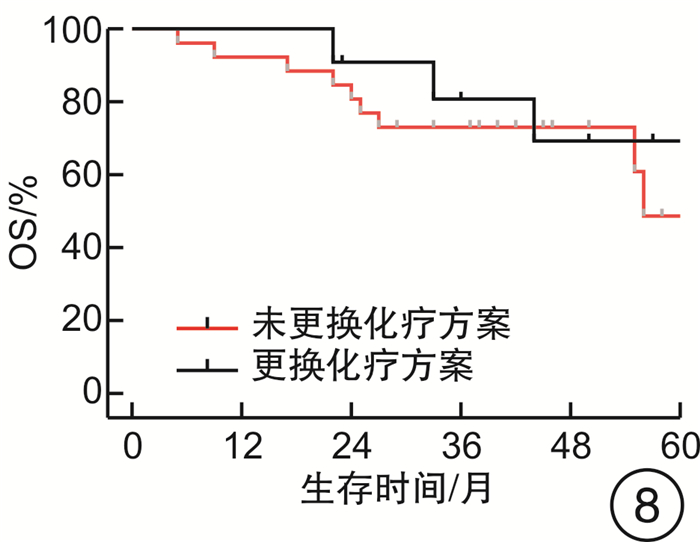
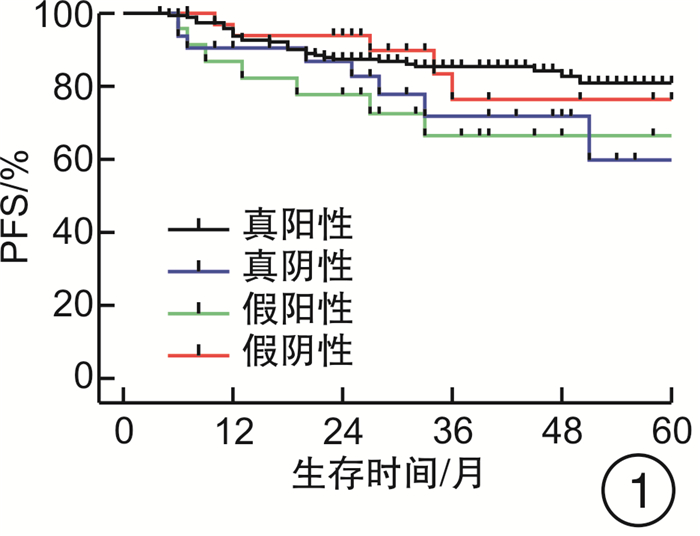
摘要: 目的 评估OncoDrug-SeqTM肿瘤精准治疗基因检测在头颈部恶性肿瘤患者中应用的临床意义。方法 回顾性分析2011年4月—2021年2月解放军总医院耳鼻咽喉头颈外科收治的局部中晚期或手术无法切除的头颈部恶性肿瘤患者338例,其中病理诊断为鳞状细胞癌301例,非鳞状细胞癌37例。所有患者均行药敏检测,根据检测结果与诱导化疗后肿瘤治疗反应情况,评估是否更换化疗方案,并计算检测的准确率及患者的生存率。结果 301例头颈部鳞状细胞癌患者,药敏检测与临床实际反应结合对比后,真阳性率、真阴性率、阳性预测值、阴性预测值、总预测准确率分别为85.37%、65.45%、91.70%、50.00%、81.73%;更换化疗方案(真阴性+假阳性)与未更换化疗方案(真阳性+假阴性)的患者5年无进展生存率(PFS)分别为63.45%和80.58%(P<0.05),5年总生存率(OS)分别为54.18%和84.74%(P<0.05)。37例头颈部非鳞状细胞癌患者的真阳性率、真阴性率、阳性预测值、阴性预测值、总预测准确率分别为88.46%、72.73%、88.46%、72.73%、83.78%;更换化疗方案与未更换化疗方案的患者5年PFS分别为68.57%和56.00%(P>0.05),5年OS分别为69.26%和48.72%(P>0.05)。结论 肿瘤药敏检测在临床医生制定个性化化疗方案中具有一定的指导意义。及时更换化疗方案,可使患者获得更为理想的疗效。相较于鳞状细胞癌,非鳞状细胞癌患者可从药敏检测中得到更大的生存获益。但由于药敏检测结果的准确性较实际情况仍存在一些偏差,因此应根据药敏检测结果结合临床实际进行决策。Abstract: Objective To evaluate the clinical significance of OncoDrug-SeqTM tumor gene detection in patients with head and neck malignancies.Methods A retrospective analysis of 338 patients with locally advanced or unresectable head and neck malignancies admitted to the Department of Otorhinolaryngology Head and Neck Surgery, Chinese PLA General Hospital from April 2011 to February 2021. Among them, 301 patients were pathologically diagnosed as squamous cell carcinoma, 37 cases were non-squamous cell carcinoma. All patients underwent OncoDrug-SeqTM gene detection, combined with the test results and the treatment response after induction chemotherapy to evaluate whether to change the chemotherapy regimen, and to calculate the accuracy of the gene detection and the survival rate of the patient.Results Among 301 patients with head and neck squamous cell carcinoma, the results of the drug sensitivity test were compared with the actual clinical response, the true positive rate(TP), true negative rate(TN), positive predictive value(PPV), negative predictive value(NPV) and total predictive accuracy rates were 85.37%, 65.45%, 91.70%, 50.00% and 81.73%, respectively. For patients who changed chemotherapy regimen(TN+FP) and did not change(TP+FN), the 5-year progression-free survival(PFS) was 63.45% and 80.58%(P < 0.05), respectively, the 5-year overall survival(OS) was 54.18% and 84.74%(P < 0.05). Among 37 patients with non-squamous cell carcinoma, the TP, TN, PPV, NPV and total predictive accuracy rates were 88.46%, 72.73%, 88.46%, 72.73%, and 83.78%, respectively. The 5-year PFS of the patients who changed(TN+FP) and did not change the treatment regimen(TP+FN) were 68.57% and 56.00%, and the 5-year OS was 69.26% and 48.72%, the difference was not statistically significant.Conclusion OncoDrug-SeqTM testing has certain significance in guiding clinicians to formulate personalized chemotherapy regimens. Timely replacement of the treatment plan can enable the patient to obtain a more ideal curative effect. Compared with patients with squamous cell carcinoma, patients with non-squamous cell carcinoma can obtain greater survival benefit from this testing. However, there are still some deviations in the accuracy of the test results compared with the actual clinical situation. Therefore, the decision should be made based on the test results in combination with the clinical reality.
-

-
表 1 338例患者的临床特征
临床特征 例数(%) χ2值 P值 临床特征 例数(%) χ2值 P值 年龄/岁 0.993 0.803 病理类型 6.426 0.377 ≤60 250(73.96) 低分化鳞癌 256(75.74) >60 88(26.04) 中分化鳞癌 27(7.99) 性别 3.144 0.370 高分化鳞癌 18(5.33) 男 260(76.92) 胚胎型横纹肌肉瘤 17(5.03) 女 78(23.08) 腺泡状横纹肌肉瘤 3(0.89) 癌种 1.633 0.652 嗅神经母细胞瘤 4(1.18) 鳞癌 301(89.05) 神经内分泌癌 3(0.89) 鼻咽鳞癌 181(53.55) 小细胞癌 2(0.59) 下咽鳞癌 54(15.98) 炎性肌纤维母细胞肉瘤 2(0.59) 口咽鳞癌 25(7.40) 恶性黑色素瘤 2(0.59) 鼻腔鼻窦鳞癌 17(5.03) 耵聍腺癌 1(0.30) 喉鳞癌 17(5.03) 多形性未分化肉瘤 1(0.30) 颈部淋巴结转移鳞癌 7(2.07) 血管肉瘤 1(0.30) 非鳞癌 37(10.95) 原始神经外胚层肿瘤 1(0.30) AJCC临床分期(第8版) 3.704 0.717 靶向治疗 0.495 0.998 Ⅱ 23(6.80) 是 254(84.39) Ⅲ 144(42.60) 否 47(15.61) Ⅳ 171(50.59) 表 2 鳞癌治疗方案
阶段 治疗方案 剂量 例数 诱导化疗 TP T:70 mg/m2,P:40 mg/m2 202 TPF/TP+TS-1 T:70 mg/m2,P:70 mg/m2,F:700 mg/m2;TS-1:40~60 mg,2/d,服2周停1周 41 G+D G:0.8~1.0 g/m2,D:20 mg/m2 58 同步放化疗 TP 同诱导化疗 16 P/奈达铂 同诱导化疗 40 T 同诱导化疗 183 G/D 同诱导化疗 58 TPF或TS-1 同诱导化疗 4 分子靶向 尼妥珠单抗/西妥昔单抗 尼妥珠单抗每周200 mg/m2,7周;或西妥昔单抗每周250 mg/m2(首剂400 mg/m2),7周 248/6 未使用 47 表 3 非鳞癌治疗方案
阶段 癌种 治疗方案 例数 诱导化疗 胚胎型横纹肌肉瘤
腺泡状横纹肌肉瘤
嗅神经母细胞瘤
神经内分泌癌
炎性肌纤维母细胞肉瘤
多形性未分化肉瘤长春地辛+IFO+多柔比星 30 恶性黑色素瘤 P+T+达卡巴嗪 2 小细胞癌 P+T+IFO 2 原始神经外胚层肿瘤 依托泊苷+IFO+P+多柔比星脂质体 1 血管肉瘤 G+T+IFO 1 耵聍腺癌 G+T 1 表 4 药敏检测结果与临床反应对比及随访结果
例 药敏结果 鳞癌 非鳞癌 例数 疾病进展 死亡 例数 疾病进展 死亡 真阳性 210 31 20 23 10 7 真阴性 36 8 9 8 2 3 假阳性 19 7 8 3 1 1 假阴性 36 5 3 3 2 2 合计 301 51 40 37 15 13 -
[1] 喻卫红, 王静, 王晓彬, 等. 恶性肿瘤患者化疗敏感性筛选的临床意义[J]. 临床肿瘤学杂志, 2013, 18(9): 799-803. doi: 10.3969/j.issn.1009-0460.2013.09.008
[2] Lau V, Wong AL, Ng C, et al. Drug sensitivity testing platforms for gastric cancer diagnostics[J]. J Clin Pathol, 2016, 69(2): 93-96. doi: 10.1136/jclinpath-2015-203426
[3] 徐航, 回翔, 朱怀军, 等. 药物基因组学在临床药物治疗中的应用[J]. 药学与临床研究, 2019, 27(1): 46-51. https://www.cnki.com.cn/Article/CJFDTOTAL-YXLY201901013.htm
[4] Pellegrino B, Cavanna L, Boggiani D, et al. Phase Ⅱ study of eribulin in combination with gemcitabine for the treatment of patients with locally advanced or metastatic triple negative breast cancer(ERIGE trial). Clinical and pharmacogenetic results on behalf of the Gruppo Oncologico Italiano di Ricerca Clinica(GOIRC)[J]. ESMO Open, 2021, 6(1): 100019. doi: 10.1016/j.esmoop.2020.100019
[5] Hofman J, Vagiannis D, Chen S, et al. Roles of CYP3A4, CYP3A5 and CYP2C8 drug-metabolizing enzymes in cellular cytostatic resistance[J]. Chem Biol Interact, 2021, 340: 109448. doi: 10.1016/j.cbi.2021.109448
[6] Formica V, Doldo E, Antonetti FR, et al. Biological and predictive role of ERCC1 polymorphisms in cancer[J]. Crit Rev Oncol Hematol, 2017, 111: 133-143. doi: 10.1016/j.critrevonc.2017.01.016
[7] Gori ar K, Kova V, Dolžan V. Clinical-pharmacogenetic models for personalized cancer treatment: application to malignant mesothelioma[J]. Sci Rep, 2017, 7: 46537. doi: 10.1038/srep46537
[8] Liao WY, Ho CC, Tsai TH, et al. Combined effect of ERCC1 and ERCC2 polymorphisms on overall survival in non-squamous non-small-cell lung cancer patients treated with first-line pemetrexed/platinum[J]. Lung Cancer, 2018, 118: 90-96. doi: 10.1016/j.lungcan.2018.01.011
[9] Zha Y, Gan P, Liu Q, et al. TP53 Codon 72 Polymorphism Predicts Efficacy of Paclitaxel Plus Capecitabine Chemotherapy in Advanced Gastric Cancer Patients[J]. Arch Med Res, 2016, 47(1): 13-18. doi: 10.1016/j.arcmed.2015.12.001
[10] Trendowski MR, El Charif O, Dinh PC Jr, et al. Genetic and Modifiable Risk Factors Contributing to Cisplatin-induced Toxicities[J]. Clin Cancer Res, 2019, 25(4): 1147-1155. doi: 10.1158/1078-0432.CCR-18-2244
[11] Matsunaga T, Kamase K, Takasawa H, et al. Facilitation of 9, 10-phenanthrenequinone-elicited neuroblastoma cell apoptosis by NAD(P)H: quinone oxidoreductase 1[J]. Chem Biol Interact, 2018, 279: 10-20. doi: 10.1016/j.cbi.2017.10.028
[12] Puerta-García E, Urbano-Pérez D, Carrasco-Campos MI, et al. Effect of DPYD, MTHFR, ABCB1, XRCC1, ERCC1 and GSTP1 on chemotherapy related toxicity in colorectal carcinoma[J]. Surg Oncol, 2020, 35: 388-398. doi: 10.1016/j.suronc.2020.09.016
[13] Wörmann B, Bokemeyer C, Burmeister T, et al. Dihydropyrimidine Dehydrogenase Testing prior to Treatment with 5-Fluorouracil, Capecitabine, and Tegafur: A Consensus Paper[J]. Oncol Res Treat, 2020, 43(11): 628-636. doi: 10.1159/000510258
[14] De Marchi P, Melendez ME, Laus AC, et al. The role of single-nucleotide polymorphism(SNPs)in toxicity of induction chemotherapy based on cisplatin and paclitaxel in patients with advanced head and neck cancer[J]. Oral Oncol, 2019, 98: 48-52. doi: 10.1016/j.oraloncology.2019.09.013
[15] Cura Y, Pérez Ramírez C, Sánchez Martín A, et al. Genetic polymorphisms on the effectiveness or safety of breast cancer treatment: Clinical relevance and future perspectives[J]. Mutat Res Rev Mutat Res, 2021, 788: 108391. doi: 10.1016/j.mrrev.2021.108391
[16] 许涛, 高远红, 陈萍, 等. 原发性喉小细胞癌六例临床分析[J]. 中华耳鼻咽喉头颈外科杂志, 2011, 46(9): 758-760. doi: 10.3760/cma.j.issn.1673-0860.2011.09.013
[17] Fang H, Langstraat CL, Visscher DW, et al. Epithelioid Inflammatory Myofibroblastic Sarcoma of the Ovary With RANB2-ALK Fusion: Report of a Case[J]. Int J Gynecol Pathol, 2018, 37(5): 468-472. doi: 10.1097/PGP.0000000000000431
[18] Young RJ, Litière S, Lia M, et al. Predictive and prognostic factors associated with soft tissue sarcoma response to chemotherapy: a subgroup analysis of the European Organisation for Research and Treatment of Cancer 62012 study[J]. Acta Oncol, 2017, 56(7): 1013-1020. doi: 10.1080/0284186X.2017.1315173
[19] Shern JF, Chen L, Chmielecki J, et al. Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors[J]. Cancer Discov, 2014, 4(2): 216-231. doi: 10.1158/2159-8290.CD-13-0639
[20] Ohgaki K, Horiuchi K, Mizutani S, et al. Primary Ewing's sarcoma/primitive neuroectodermal tumor of the kidney that responded to low-dose chemotherapy with ifosfamide, etoposide, and doxorubicin[J]. Int J Clin Oncol, 2010, 15(2): 210-214. doi: 10.1007/s10147-010-0031-3
[21] Friedman AA, Letai A, Fisher DE, et al. Precision medicine for cancer with next-generation functional diagnostics[J]. Nat Rev Cancer, 2015, 15(12): 747-756. doi: 10.1038/nrc4015
[22] Walko C, Kiel PJ, Kolesar J. Precision medicine in oncology: New practice models and roles for oncology pharmacists[J]. Am J Health Syst Pharm, 2016, 73(23): 1935-1942. doi: 10.2146/ajhp160211
[23] Kondo T, Kubota T, Tanimura H, et al. Cumulative results of chemosensitivity tests for antitumor agents in Japan. Japan Research Society for Appropriate Cancer Chemotherapy[J]. Anticancer Res, 2000, 20(4): 2389-2392.
[24] Fruehauf JP. In vitro assay-assisted treatment selection for women with breast or ovarian cancer[J]. Endocr Relat Cancer, 2002, 9(3): 171-182.
[25] Hwu P, Bedikian AY, Grimm EA. Challenges of chemosensitivity testing[J]. Clin Cancer Res, 2006, 12(18): 5258-5259. doi: 10.1158/1078-0432.CCR-06-1656
[26] Yang C, Yang C, Yarden Y, et al. The prospects of tumor chemosensitivity testing at the single-cell level[J]. Drug Resist Updat, 2021, 54: 100741. doi: 10.1016/j.drup.2020.100741
-




 下载:
下载: