-
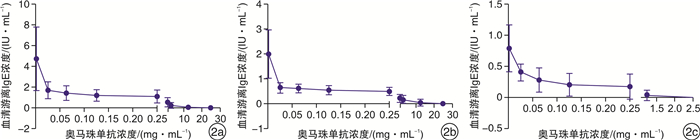
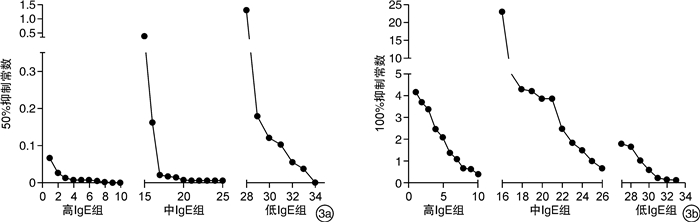
摘要: 目的 研究奥马珠单抗与个体游离免疫球蛋白E(IgE)的结合能力强弱及其对奥马珠单抗作用的影响。方法 收集变应性鼻炎患者的血清共28份,分为高、中、低IgE组。在体外给予不同剂量的奥马珠单抗进行抑制性结合,应用酶联免疫吸附试验检测抑制后血清中游离IgE的变化,计算奥马珠单抗对血清游离IgE的抑制常数,同时分析奥马珠单抗与个体游离IgE结合能力情况。结果 从总体趋势来看,当对血清中100%游离IgE进行抑制时,所需加入的奥马珠单抗与原血清中游离IgE浓度呈正相关(高、中、低IgE组所需奥马珠单抗浓度分别为9.500±7.207、8.636±7.375、0.786±0.857)。而对血清中50%游离IgE进行抑制时,所需要的奥马珠单抗剂量明显更低(高、中、低IgE组所需奥马珠单抗浓度分别为0.049±0.071、0.046±0.077、0.048±0.048)。而不同个体血清游离IgE的100%及50%抑制常数均存在一定程度的差异。结论 从总的趋势看,要达到同样的抑制效果,奥马珠单抗的需要剂量与IgE浓度成正比。对于部分敏感患者,即使给予小于推荐剂量的奥马珠单抗,亦可出现游离IgE被部分结合抑制。不同个体血清游离IgE与奥马珠单抗的结合力存在差异。临床用药若将此因素作为奥马珠个体化使用剂量的参考,将有可能更加精准地达到预期疗效。Abstract: Objective To investigate the individual binding ability of omalizumab to free IgE and its effect on omalizumab action.Methods A total of 28 serum samples were collected from patients with allergic rhinitis and divided into groups with high, medium and low concentrations of free IgE. Different doses of omalizumab were administered in vitro for inhibitory binding. Enzyme linked immunosorbent assay was used to detect changes in serum free IgE after inhibition. The inhibition constant of omalizumab on serum free IgE was calculated. At the same time, the binding ability of omalizumab and individual free IgE was analyzed.Results In general, when 100% serum free IgE was inhibited, the omalizumab required was positively correlated with the original serum free IgE concentration(9.500±7.207, 8.636±7.375, and 0.786±0.857 for the high, medium, and low IgE concentration groups, respectively). The dose of omalizumab required for inhibition of 50% free IgE in serum was significantly lower(0.049±0.071, 0.046±0.077, 0.048±0.048 in the high, medium, and low IgE concentrations groups, respectively). The 100% and 50% inhibition constants of serum free IgE in different individuals were different to some extent.Conclusion Overall, the amount of omalizumab required to achieve the same inhibitory effect is proportional to the IgE concentration. In some sensitive patients, partial binding inhibition of free IgE can occur even when omalizumab is administered at less than the recommended dose. The binding ability of serum free IgE and omalizumab was different in different individuals. If this factor is used as a reference for the individual dose of omalizumab in clinical medicine, it is possible to achieve the expected efficacy more accurately.
-
Key words:
- rhinitis, allergic /
- omalizumab /
- free immunoglobulin E /
- individual binding ability
-

-
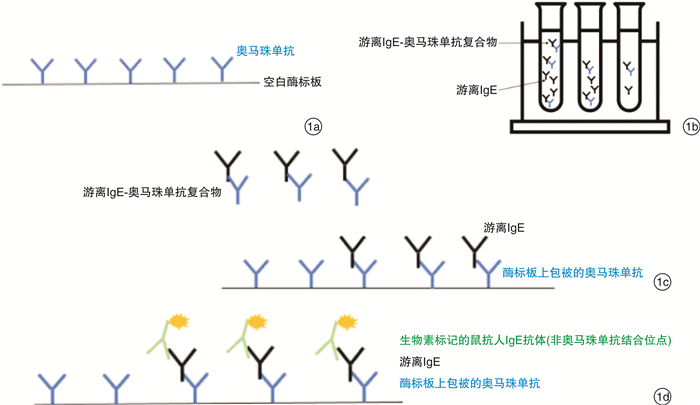
表 1 游离IgE测定的实验原理
mg/mL,x±s 项目 高IgE组 中IgE组 低IgE组 P 抑制50%游离IgE 0.049±0.071 0.046±0.077 0.048±0.048 0.996 抑制100%游离IgE 9.500±7.207 8.636±7.375 0.786±0.857 0.030 -
[1] Cardet JC, Casale TB. New insights into the utility of omalizumab[J]. J Allergy Clin Immunol, 2019, 143(3): 923-926.e1. doi: 10.1016/j.jaci.2019.01.016
[2] Hamilton RG, Marcotte GV, Saini SS. Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving omalizumab(Xolair)therapy[J]. J Immunol Methods, 2005, 303(1/2): 81-91.
[3] Schreiber J, Schwab Sauerbeck I, Mailänder C. The Long-Term Effectiveness and Safety of Omalizumab on Patient-and Physician-Reported Asthma Control: A Three-Year, Real-Life Observational Study[J]. Adv Ther, 2020, 37(1): 353-363. doi: 10.1007/s12325-019-01135-w
[4] Lapeere H, Baeck M, Stockman A, et al. A retrospective analysis omalizumab treatment patterns in patients with chronic spontaneous urticaria: a real-world study in Belgium[J]. J Eur Acad Dermatol Venereol, 2020, 34(1): 127-134. doi: 10.1111/jdv.15684
[5] Metz M, Vadasz Z, Kocatürk E, et al. Omalizumab Updosing in Chronic Spontaneous Urticaria: an Overview of Real-World Evidence[J]. Clin Rev Allergy Immunol, 2020, 59(1): 38-45. doi: 10.1007/s12016-020-08794-6
[6] Humbert M, Bousquet J, Bachert C, et al. IgE-Mediated Multimorbidities in Allergic Asthma and the Potential for Omalizumab Therapy[J]. J Allergy Clin Immunol Pract, 2019, 7(5): 1418-1429. doi: 10.1016/j.jaip.2019.02.030
[7] Gevaert P, Omachi TA, Corren J, et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials[J]. J Allergy Clin Immunol, 2020, 146(3): 595-605. doi: 10.1016/j.jaci.2020.05.032
[8] Brandström J, Vetander M, Sundqvist AC, et al. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents[J]. Clin Exp Allergy, 2019, 49(10): 1328-1341. doi: 10.1111/cea.13469
[9] Okayama Y, Matsumoto H, Odajima H, et al. Roles of omalizumab in various allergic diseases[J]. Allergol Int, 2020, 69(2): 167-177. doi: 10.1016/j.alit.2020.01.004
[10] Altrichter S, Chuamanochan M, Knoth H, et al. Real-life treatment of cholinergic urticaria with omalizumab[J]. J Allergy Clin Immunol, 2019, 143(2): 788-791.e8. doi: 10.1016/j.jaci.2018.08.050
[11] Mendoza Alvarez LB, Barker R, Nelson C, et al. Clinical response to omalizumab in patients with hereditary α-tryptasemia[J]. Ann Allergy Asthma Immunol, 2020, 124(1): 99-100.e1. doi: 10.1016/j.anai.2019.09.026
[12] Berry R, Hollingsworth P, Lucas M. Successful treatment of idiopathic mast cell activation syndrome with low-dose Omalizumab[J]. Clin Transl Immunology, 2019, 8(10): e01075.
[13] Kafi P, Edén I, Swartling C. Morbihan Syndrome Successfully Treated with Omalizumab[J]. Acta Derm Venereol, 2019, 99(7): 677-678. doi: 10.2340/00015555-3168
[14] Casale TB, Bernstein IL, Busse WW, et al. Use of an anti-IgE humanized mono-clonal antibody in ragweed-induced allergic rhinitis[J]. Allergy Clin Immunol, 1997, 100(1): 110-121. doi: 10.1016/S0091-6749(97)70202-1
[15] Verschoor D, von Gunten S. Allergy and Atopic Diseases: An Update on Experimental Evidence[J]. Int Arch Allergy Immunol, 2019, 180(4): 235-243. doi: 10.1159/000504439
[16] 马婷婷, 王向东, 陈艳蕾, 等. 奥马珠单抗治疗花粉症伴中重度哮喘患者的临床疗效及安全性研究[J]. 中国耳鼻咽喉头颈外科, 2018, 25(12): 675-678. https://www.cnki.com.cn/Article/CJFDTOTAL-EBYT201812013.htm
[17] Ren L, Zhang N, Zhang L, et al. Biologics for the treatment of chronic rhinosinusitis with nasal polyps-state of the art[J]. World Allergy Organ J, 2019, 12(8): 100050. doi: 10.1016/j.waojou.2019.100050
[18] 高培, 余文婷, 周玥, 等. 奥马珠单抗联合RIT与糖皮质激素联合RIT的安全性比较[J]. 临床耳鼻咽喉头颈外科杂志, 2020, 34(7): 610-614. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH202007009.htm
[19] Rivière GJ, Yeh CM, Reynolds CV, et al. Bioequivalence of a Novel Omalizumab Solution for Injection Compared with the Standard Lyophilized Powder Formulation[J]. Bioequiv Availab, 2011, 3(6): 144-150.
[20] Holgate S, Casale T, Wenzel S, et al. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation[J]. J Allergy Clin Immunol, 2005, 115(3): 459-465. doi: 10.1016/j.jaci.2004.11.053
[21] Lichtenstein LM, Kagey-Sobotka A, White JM, et al. Anti-human IgG causes basophil histamine release by acting on IgG-IgE complexes bound to IgE receptors[J]. J Immunol, 1992, 148(12): 3929-3936.
[22] Ritter C, Bättig M, Kraemer R, et al. IgE hidden in immune complexes with anti-IgE autoantibodies in children with asthma[J]. J Allergy Clin Immunol, 1991, 88(5): 793-801. doi: 10.1016/0091-6749(91)90187-S
[23] MacGlashan D Jr. Therapeutic efficacy of omalizumab[J]. J Allergy Clin Immunol, 2009, 123(1): 114-115. doi: 10.1016/j.jaci.2008.10.053
-

| 引用本文: | 高培, 祝婉婷, 张启迪, 等. 奥马珠单抗与游离IgE结合力的离体实验研究[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(12): 1063-1068. doi: 10.13201/j.issn.2096-7993.2021.12.002 |
| Citation: | GAO Pei, ZHU Wanting, ZHANG Qidi, et al. In vitro study on the individual binding ability of omalizumab with free IgE[J]. J Clin Otorhinolaryngol Head Neck Surg, 2021, 35(12): 1063-1068. doi: 10.13201/j.issn.2096-7993.2021.12.002 |
- Figure 1.
- Figure 2.
- Figure 3.



 下载:
下载: