Endothelial cells and fibroblasts mediate the microenvironmental regulatory network of carotid body paraganglioma
-
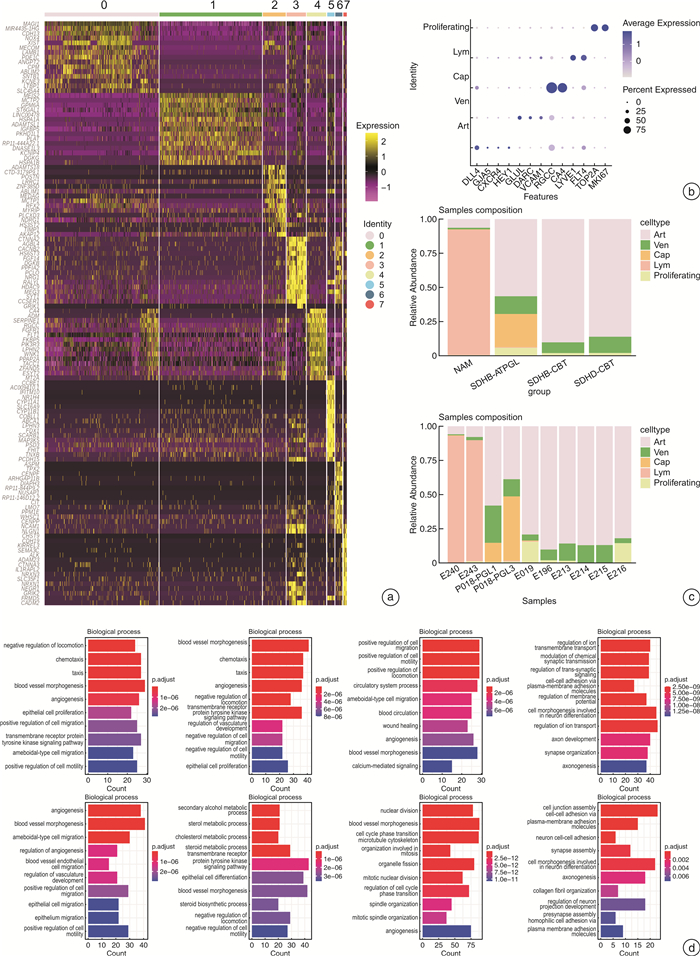
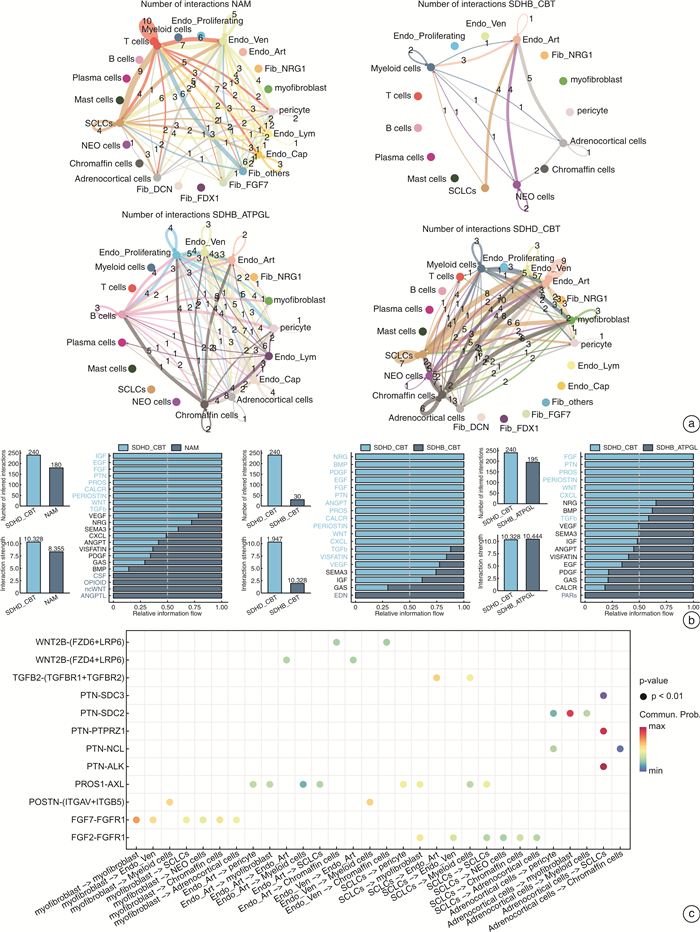
摘要: 目的 探讨SDHD突变的颈动脉体副神经节瘤(SDHD-CBT)微环境中内皮细胞和成纤维细胞的基因表达特征,精细各亚群的功能富集情况,进一步探索SDHD-CBT微环境中细胞间相互调控网络。方法 采用生物信息学分析手段,下载并重新分析SDHD-CBT、SDHB突变的胸腹部副神经节瘤(SDHB-ATPGL)、SDHB-CBT和正常肾上腺髓质(NAM)的单细胞核RNA测序数据,明确样本的细胞群信息,重点探索内皮细胞和成纤维细胞亚群的基因表达特征,使用GO富集分析对亚群进行功能富集,并运用CellChat比较不同临床样本的细胞间相互调控网络,预测SDHD-CBT中的显著通路。结果 共获得7个细胞群,SDHD-CBT内皮细胞主要亚群是动脉和静脉内皮细胞,成纤维细胞主要亚群是肌成纤维细胞和周细胞。相比于NAM,SDHB-CBT和SDHB-ATPGL,SDHD-CBT中内皮细胞和成纤维细胞参与的细胞通讯更加丰富,显著富集FGF、PTN、WNT、PROS、PERIOSTIN和TGFb等通路。结论 SDHD-CBT中内皮细胞和成纤维细胞具有异质性,参与重要细胞通讯过程,其中,FGF、PTN、WNT、PROS、PERIOSTIN和TGFb通路的发现可能在SDHD-CBT微环境调控研究中起重要作用。Abstract: Objective To explore the gene expression characteristics of endothelial cells and fibroblasts in the microenvironment of SDHD-mutated carotid body tumors(SDHD-CBT), to fine the functional enrichment of each subcluster, and to further explore the network of cell-cell interactions in the microenvironment of SDHD-CBT.Methods The bioinformatics analysis was used to download and reanalyze the single-nuclear RNA sequencing data of SDHD-CBT, SDHB mutated thoracic and abdominal paraganglioma(SDHB-ATPGL), SDHB-CBT, and normal adrenal medulla(NAM), to clarify the information of cell populations of the samples. We focused on exploring the gene expression profiles of endothelial cells and fibroblasts subclusters, and performed functional enrichment analysis based on Gene Ontology(GO) resources. CellChat was used to compare the cell-cell interactions networks of different clinical samples and predict significant signaling pathways in SDHD-CBT.Results A total of 7 cell populations were profiled. The main subtypes of endothelial cells in SDHD-CBT are arterial and venous endothelial cells, and the main subtypes of fibroblasts are myofibroblasts and pericytes. Compared to NAM, SDHB-CBT and SDHB-ATPGL, cell communication involving endothelial cells and fibroblasts in SDHD-CBT is more abundant, with significant enrichment in pathways such as FGF, PTN, WNT, PROS, PERIOSTIN, and TGFb.Conclusion Endothelial cells and fibroblasts in SDHD-CBT are heterogeneous and involved in important cellular interactionprocesses, in which the discovery of FGF, PTN, WNT, PROS, PERIOSTIN and TGFb signals may play an important role in the regulation of microenvironment of SDHD-CBT.
-

-
[1] Aygun N, Uludag M. Pheochromocytoma and paraganglioma: from epidemiology to clinical findings[J]. Sisli Etfal Hastan Tip Bul, 2020, 54(2): 159-168.
[2] Fliedner SMJ, Brabant G, Lehnert H. Pheochromocytoma and paraganglioma: genotype versus anatomic location as determinants of tumor phenotype[J]. Cell Tissue Res, 2018, 372(2): 347-365. doi: 10.1007/s00441-017-2760-3
[3] Woolen S, Gemmete JJ. Paragangliomas of the head and neck[J]. Neuroimaging Clin N Am, 2016, 26(2): 259-278. doi: 10.1016/j.nic.2015.12.005
[4] Valero C, Ganly I, Shah JP. Head and neck paragangliomas: 30-year experience[J]. Head Neck, 2020, 42(9): 2486-2495. doi: 10.1002/hed.26277
[5] 吕海丽, 李谱, 张名霞, 等. 颈动脉体瘤术后16年发生颈部淋巴结转移1例[J]. 临床耳鼻咽喉头颈外科杂志, 2022, 36(6): 475-476. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2022.06.014
[6] Cleere EF, Martin-Grace J, Gendre A, et al. Contemporary management of paragangliomas of the head and neck[J]. Laryngoscope Investig Otolaryngol, 2022, 7(1): 93-107. doi: 10.1002/lio2.706
[7] van Hulsteijn LT, Dekkers OM, Hes FJ, et al. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis[J]. J Med Genet, 2012, 49(12): 768-776. doi: 10.1136/jmedgenet-2012-101192
[8] Ricketts CJ, Forman JR, Rattenberry E, et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD[J]. Hum Mutat, 2010, 31(1): 41-51. doi: 10.1002/humu.21136
[9] Taïeb D, Nölting S, Perrier ND, et al. Management of phaeochromocytoma and paraganglioma in patients with germline SDHB pathogenic variants: an international expert Consensus statement[J]. Nat Rev Endocrinol, 2024, 20(3): 168-184. doi: 10.1038/s41574-023-00926-0
[10] Barbolosi D, Crona J, Serre R, et al. Mathematical modeling of disease dynamics in SDHB-and SDHD-related paraganglioma: Further step in understanding hereditary tumor differences and future therapeutic strategies[J]. PLoS One, 2018, 13(8): e0201303. doi: 10.1371/journal.pone.0201303
[11] Kuo CC, Wu JY, Wu KK. Cancer-derived extracellular succinate: a driver of cancer metastasis[J]. J Biomed Sci, 2022, 29(1): 93. doi: 10.1186/s12929-022-00878-z
[12] Zethoven M, Martelotto L, Pattison A, et al. Single-nuclei and bulk-tissue gene-expression analysis of pheochromocytoma and paraganglioma links disease subtypes with tumor microenvironment[J]. Nat Commun, 2022, 13: 6262. doi: 10.1038/s41467-022-34011-3
[13] Zhang X, Lian P, Su M, et al. Single-cell transcriptome analysis identifies a unique tumor cell type producing multiple hormones in ectopic ACTH and CRH secreting pheochromocytoma[J]. Elife, 2021, 10: e68436. doi: 10.7554/eLife.68436
[14] Kalucka J, de Rooij LPMH, Goveia J, et al. Single-cell transcriptome atlas of murine endothelial cells[J]. Cell, 2020, 180(4): 764-779. e20. doi: 10.1016/j.cell.2020.01.015
[15] Muhl L, Genové G, Leptidis S, et al. Single-cell analysis uncovers fibroblast heterogeneity and criteria for fibroblast and mural cell identification and discrimination[J]. Nat Commun, 2020, 11(1): 3953. doi: 10.1038/s41467-020-17740-1
[16] Sathe A, Mason K, Grimes SM, et al. Colorectal cancer metastases in the liver establish immunosuppressive spatial networking between tumor-associated SPP1+ macrophages and fibroblasts[J]. Clin Cancer Res, 2023, 29(1): 244-260. doi: 10.1158/1078-0432.CCR-22-2041
[17] Hanley CJ, Waise S, Ellis MJ, et al. Single-cell analysis reveals prognostic fibroblast subpopulations linked to molecular and immunological subtypes of lung cancer[J]. Nat Commun, 2023, 14: 387. doi: 10.1038/s41467-023-35832-6
[18] Cords L, Tietscher S, Anzeneder T, et al. Cancer-associated fibroblast classification in single-cell and spatial proteomics data[J]. Nat Commun, 2023, 14: 4294. doi: 10.1038/s41467-023-39762-1
[19] Buffet A, Burnichon N, Favier J, et al. An overview of 20years of genetic studies in pheochromocytoma and paraganglioma[J]. Best Pract Res Clin Endocrinol Metab, 2020, 34(2): 101416. doi: 10.1016/j.beem.2020.101416
[20] Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations[J]. JAMA, 2004, 292(8): 943-951. doi: 10.1001/jama.292.8.943
[21] Richter S, D'Antongiovanni V, Martinelli S, et al. Primary fibroblast co-culture stimulates growth and metabolism in Sdhb-impaired mouse pheochromocytoma MTT cells[J]. Cell Tissue Res, 2018, 374(3): 473-485. doi: 10.1007/s00441-018-2907-x
[22] Gudgeon N, Munford H, Bishop EL, et al. Succinate uptake by T cells suppresses their effector function via inhibition of mitochondrial glucose oxidation[J]. Cell Rep, 2022, 40(7): 111193. doi: 10.1016/j.celrep.2022.111193
[23] Tufton N, Hearnden RJ, Berney DM, et al. The immune cell infiltrate in the tumour microenvironment of phaeochromocytomas and paragangliomas[J]. Endocr Relat Cancer, 2022, 29(11): 589-598.
[24] De Fabritiis S, Valentinuzzi S, Piras G, et al. Targeted metabolomics detects a putatively diagnostic signature in plasma and dried blood spots from head and neck paraganglioma patients[J]. Oncogenesis, 2023, 12(1): 10. doi: 10.1038/s41389-023-00456-4
[25] Pang LZ, Dunterman M, Xuan WJ, et al. Circadian regulator CLOCK promotes tumor angiogenesis in glioblastoma[J]. Cell Rep, 2023, 42(2): 112127. doi: 10.1016/j.celrep.2023.112127
[26] Qin PF, Chen HX, Wang YH, et al. Cancer-associated fibroblasts undergoing neoadjuvant chemotherapy suppress rectal cancer revealed by single-cell and spatial transcriptomics[J]. Cell Rep Med, 2023, 4(10): 101231. doi: 10.1016/j.xcrm.2023.101231
[27] Zhang W, Ge Y, Cheng Q, et al. Decorin is a pivotal effector in the extracellular matrix and tumour microenvironment[J]. Oncotarget, 2018, 9(4): 5480-5491. doi: 10.18632/oncotarget.23869
[28] Wang SX, Qu YK, Fang X, et al. Decorin: a potential therapeutic candidate for ligamentum flavum hypertrophy by antagonizing TGF-β1[J]. Exp Mol Med, 2023, 55(7): 1413-1423. doi: 10.1038/s12276-023-01023-y
[29] Ping QR, Wang CH, Cheng X, et al. TGF-β1 dominates stromal fibroblast-mediated EMT via the FAP/VCAN axis in bladder cancer cells[J]. J Transl Med, 2023, 21(1): 475. doi: 10.1186/s12967-023-04303-3
[30] Yue HR, Li WZ, Chen RF, et al. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer[J]. Gynecol Oncol, 2021, 160(2): 530-538. doi: 10.1016/j.ygyno.2020.11.026
[31] Hu Y, Recouvreux MS, Haro M, et al. INHBA(+)cancer-associated fibroblasts generate an immunosuppressive tumor microenvironment in ovarian cancer[J]. NPJ Precis Oncol, 2024, 8(1): 35. doi: 10.1038/s41698-024-00523-y
[32] Li ZY, Pai R, Gupta S, et al. Presence of onco-fetal neighborhoods in hepatocellular carcinoma is associated with relapse and response to immunotherapy[J]. Nat Cancer, 2024, 5(1): 167-186. doi: 10.1038/s43018-023-00672-2
[33] Martin A, Venara M, Mathó C, et al. Fibroblast deficiency of insulin-like growth factor 1 receptor type 1(IGF1R)impairs initial steps of murine pheochromocytoma development[J]. Biochimie, 2019, 163: 108-116. doi: 10.1016/j.biochi.2019.06.004
[34] Fernandez MC, Martin A, Venara M, et al. Overexpression of the insulin-like growth factor 1 receptor(IGF-1R)is associated with malignancy in familial pheochromocytomas and paragangliomas[J]. Clin Endocrinol, 2013, 79(5): 623-630. doi: 10.1111/cen.12205
[35] Qin S, Xu YW, Yu SM, et al. Molecular classification and tumor microenvironment characteristics in pheochromocytomas[J]. Elife, 2024, 12: RP87586. doi: 10.7554/eLife.87586
[36] Katoh M. Therapeutics targeting FGF signaling network in human diseases[J]. Trends Pharmacol Sci, 2016, 37(12): 1081-1096. doi: 10.1016/j.tips.2016.10.003
-





 下载:
下载: