Molecular allergen IgE tests show neosensitizaions occurring during house dust mite specific immunotherapy
-
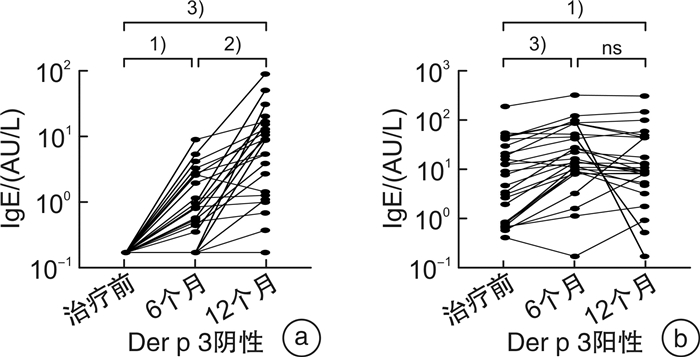
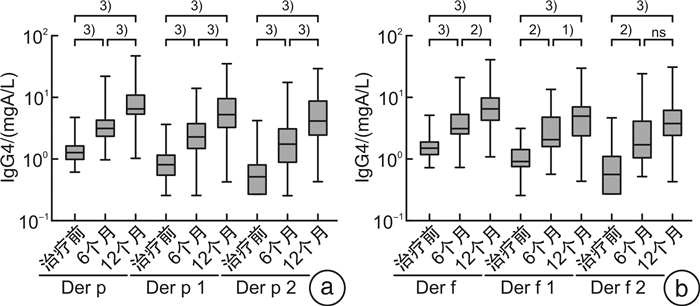
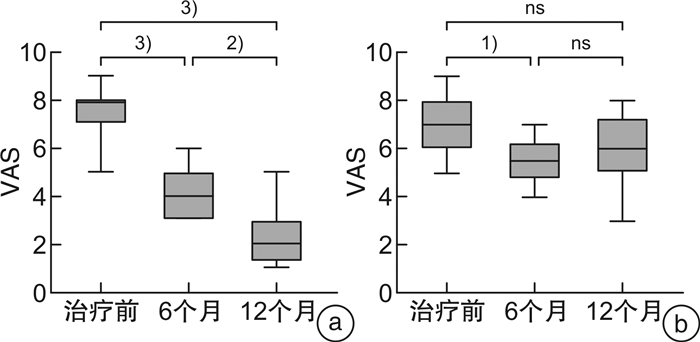
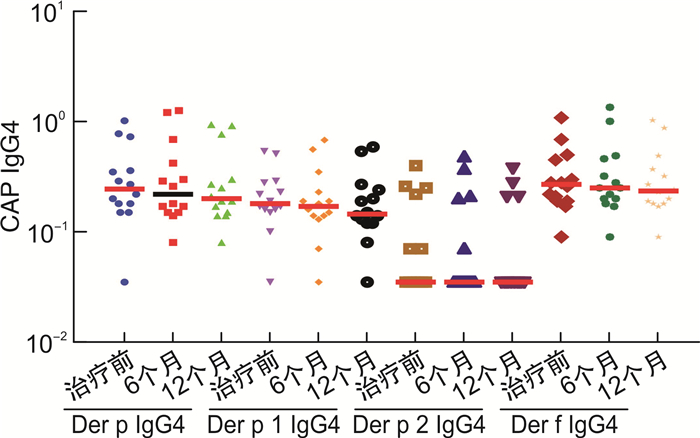
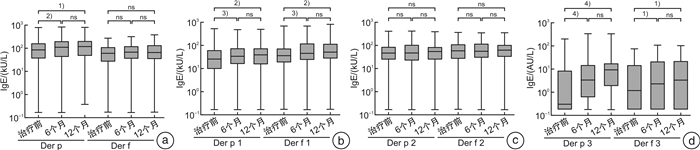
摘要: 目的 由于患者过敏原分子致敏特征和脱敏制剂组成的差异,患者可能在过敏原特异性免疫治疗中产生新的致敏。本研究探讨屋尘螨特异性免疫治疗是否会产生新的致敏,以及新的致敏的临床重要性。方法 53例尘螨引起的过敏性鼻炎伴随或不伴随哮喘患者接受为期1年的免疫治疗,14例患者作为对照组,按需接受对症药物治疗。分别在治疗前、治疗6个月及12个月采集患者血清,检测屋尘螨、粉尘螨及其组分过敏原(Der p 1/2/3和Der f 1/2/3)特异性IgE抗体及IgG4抗体水平(Der p,Der p 1/2,Der f,Der f 1/2),同时收集3个时间点的VAS评分。结果 免疫治疗1年后免疫治疗组患者Der p,Der p1/3及Der f 1/3特异性IgE抗体水平显著增高,尤其是Derp 3,其中69.2%(18/26)治疗前该过敏原阴性的患者转为阳性即产生新增过敏。免疫治疗组患者过敏原特异性IgG4抗体浓度1年后显著增高,VAS评分显著下降。对照组特异性IgE及IgG4抗体浓度及VAS评分和治疗前比较,无显著变化。新增致敏的患者和未新增致敏的患者比较,IgG4抗体浓度和VAS评分无显著性差异。结论 屋尘螨特异性免疫治疗产生了新的致敏,但其临床重要性并不清楚,有待进一步研究。Abstract: Objective Neosensitizations may be occur during the allergen specific immunotherapy(AIT) due to the differences between allergen vaccine's content and a patient's molecular sensitization profile. This study investigates whether AIT with HDM extract changes the sensitization profile, whether de novo sensitization occurs, and the clinical importance of the neosensitization.Methods Fifty-three patients with HDM allergic rhinitis, with/without asthma, patients were received one year HDM subcutaneous AIT. Fourteen patients were recruited as control group and received only necessary medications. Serum samples were collected at baseline, 6thmoths and 12thof AIT, respectively. Serum samples were tested specific IgE against Der p, Der p 1/2/3 and Der f, Der f 1/2/3, as well as IgG4 against Der p, Der p 1/2 and Der f, Der f 1/2. VAS were collected at the time-points as well.Results In AIT group, Der p, Der p 1/3, and Der f 1/3 specific IgE levels were significantly higher after one-year treatment, especially for Der p 3. There were 69.2%(18/26) patients whose Der p 3 specific IgE below 0.35 kU/L at baseline but became positive(>0.35 kU/L) after treatment, that is, neosensitization occurred. All tested allergen specific IgG4 level significantly increased after one year AIT treatment and the VAS declined dramatically. However, for patients with neosensitization and without neosensitization, there were no significantly changes concerning to IgG4 level and VAS.Conclusion Patients undergoing AIT might have a risk of neosensitization to the allergen components in the vaccines. However, the clinical importance of the neosensitization remains unclear and warrants further studies.
-
Key words:
- allergen specific immunotherapy /
- house dust mite /
- add sensitization /
- IgE /
- IgG4 /
- molecular sensitization spectrum
-

-
表 1 AIT治疗前免疫治疗组患者特异性水平
序号 Der p Der p 1 Der p 2 Der p 3 Der f Der f 1 Der f 2 Der f 3 1 632.19 540.51 265.93 0.17 218.59 545.26 323.21 0.17 2 24.17 7.75 10.21 0.17 25.73 19.86 13.16 4.36 3 91.94 25.90 60.82 0.17 62.34 18.03 57.66 0.17 4 245.34 99.11 101.90 48.25 120.63 107.96 95.23 49.62 5 142.08 72.98 106.41 12.93 83.25 77.08 151.02 19.80 6 178.03 157.43 27.87 45.28 123.90 179.21 121.14 43.29 7 49.12 21.46 31.33 0.17 56.16 58.84 49.41 0.17 8 39.35 48.12 0.17 0.17 103.69 85.31 0.17 0.17 9 50.60 13.54 34.32 0.84 36.98 21.74 48.65 6.34 10 35.68 17.31 27.59 0.17 24.84 37.16 63.99 3.63 11 152.04 55.35 70.90 18.55 103.33 60.83 74.55 23.31 12 644.33 439.83 399.76 188.84 275.85 700.79 356.60 69.64 13 89.51 8.53 72.72 0.17 133.46 38.92 116.07 3.66 14 371.24 176.35 178.18 0.17 141.36 46.13 135.39 0.17 15 117.56 19.68 76.54 0.68 159.23 34.46 125.10 0.60 16 32.09 12.39 87.12 0.17 74.29 77.34 124.52 28.61 17 4.82 0.63 11.36 0.17 13.39 7.27 19.18 0.17 18 35.85 28.41 42.15 0.64 59.91 57.66 44.02 0.17 19 4.59 0.17 4.83 0.17 3.65 0.90 7.61 0.17 20 200.78 134.02 99.04 44.06 142.68 195.50 95.70 1.83 21 13.71 0.52 27.74 0.17 32.08 9.00 57.88 1.22 22 319.41 159.45 182.74 7.74 234.34 230.02 208.34 33.06 23 150.96 42.29 50.72 0.41 99.08 31.52 51.61 0.68 24 103.47 26.05 32.25 54.15 59.37 24.35 48.09 22.68 25 37.59 5.00 29.26 0.17 52.77 32.07 34.93 2.04 26 105.17 27.81 30.61 0.58 79.82 36.01 29.26 3.11 27 44.68 18.91 75.80 0.17 65.30 27.46 92.67 12.83 28 16.68 7.15 3.72 0.17 11.89 10.44 5.01 0.17 29 1.40 0.17 0.17 0.17 0.54 0.17 0.17 0.17 30 70.46 13.02 57.89 0.17 58.27 44.24 62.56 5.04 31 54.29 21.65 10.45 1.95 22.72 26.43 11.12 2.18 32 165.73 234.50 0.17 0.17 18.60 150.85 0.17 0.17 33 52.14 4.00 73.19 0.17 84.11 10.22 79.12 2.33 34 64.55 22.81 29.20 2.64 36.85 35.26 31.00 0.37 35 7.49 6.98 0.17 0.17 0.53 5.39 0.17 0.17 36 199.86 62.38 53.74 9.13 36.36 21.05 76.27 0.61 37 224.63 75.64 114.42 29.77 174.02 113.46 107.05 40.02 38 12.22 3.59 14.98 0.71 15.24 6.30 22.50 1.23 39 622.77 140.99 143.29 0.81 132.49 204.69 124.54 0.17 40 33.60 10.07 29.48 0.17 44.34 19.06 32.18 0.91 41 797.53 51.66 129.24 3.09 247.32 138.35 118.01 33.03 42 0.61 0.17 0.17 0.17 0.17 1.23 0.17 0.17 43 81.95 15.30 26.53 0.17 57.75 18.54 26.24 0.17 44 351.40 97.80 98.64 12.54 87.71 74.80 125.48 0.17 45 220.16 49.55 55.07 4.70 112.97 74.74 74.94 3.40 46 175.84 135.32 65.63 0.78 47.62 69.44 70.49 0.17 47 131.54 54.87 41.65 0.17 44.96 30.85 38.50 0.17 48 139.47 32.33 96.25 21.06 24.75 10.35 113.68 1.07 49 125.14 32.74 51.90 0.17 70.90 36.64 54.43 0.17 50 78.43 47.07 92.09 0.17 188.46 140.59 133.35 62.35 51 137.94 29.19 61.64 41.35 75.37 48.59 74.47 27.08 52 8.15 11.40 0.17 3.43 1.26 11.72 0.17 0.17 53 80.28 20.40 35.33 15.96 61.58 53.53 43.18 47.52 阳性率/% 100.00 94.30 88.70 50.90 98.10 98.10 88.70 62.30 中位数 89.51 26.05 50.72 0.41 61.58 36.64 57.88 1.22 -
[1] Agache I, Lau S, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: House dust mite-driven allergic asthma[J]. Allergy, 2019, 74(5): 855-873. doi: 10.1111/all.13749
[2] Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper[J]. Allergy, 2017, 72(8): 1156-1173. doi: 10.1111/all.13138
[3] Bao YX, Chen JJ, Cheng L, et al. Chinese Guideline on allergen immunotherapy for allergic rhinitis[J]. J Thorac Dis, 2017, 9(11): 4607-4650. doi: 10.21037/jtd.2017.10.112
[4] Farraia M, Paciência I, Castro Mendes F, et al. Allergen immunotherapy for asthma prevention: a systematic review and meta-analysis of randomized and non-randomized controlled studies[J]. Allergy, 2022, 77(6): 1719-1735. doi: 10.1111/all.15295
[5] Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy[J]. Trends Mol Med, 2010, 16(7): 321-328. doi: 10.1016/j.molmed.2010.04.008
[6] Wang WJ, Wang JH, Song GH, et al. Environmental and sensitization variations among asthma and/or rhinitis patients between 2008 and 2018 in China[J]. Clin Transl Allergy, 2022, 12(2): e12116. doi: 10.1002/clt2.12116
[7] Li J, Sun B, Huang Y, et al. A multicentre study assessing the prevalence of sensitizations in patients with asthma and/or rhinitis in China[J]. Allergy, 2009, 64(7): 1083-1092. doi: 10.1111/j.1398-9995.2009.01967.x
[8] Nittner-Marszalska M, Kopeć A, Foks-Ciekalska A, et al. Monitoring of molecular profiling of allergen-antibody responses in HDM-immunotherapy patients[J]. Hum Vaccin Immunother, 2022, 18(7): 2148815. doi: 10.1080/21645515.2022.2148815
[9] 中华耳鼻咽喉头颈外科杂志编辑委员会鼻科组, 中华医学会耳鼻咽喉头颈外科学分会鼻科学组. 变应性鼻炎诊断和治疗指南(2015年, 天津)[J]. 中华耳鼻咽喉头颈外科杂志, 2016, 51(1): 6-24. https://xuewen.cnki.net/CCND-HJRB202406130011.html
[10] 陈浩, 纪平, 杨林, 等. 尘螨诱发的变应性鼻炎患者的组分特征[J]. 临床耳鼻咽喉头颈外科杂志, 2023, 37(6): 442-447. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2023.06.007
[11] Zhao L, Zhang Y, Zhang S, et al. The effect of immunotherapy on cross-reactivity between house dust mite and other allergens in house dust mite-sensitized patients with allergic rhinitis[J]. Expert Rev Clin Immunol, 2021, 17(9): 969-975. doi: 10.1080/1744666X.2021.1968834
[12] Rodríguez-Domínguez A, Berings M, Rohrbach A, et al. Molecular profiling of allergen-specific antibody responses may enhance success of specific immunotherapy[J]. J Allergy Clin Immunol, 2020, 146(5): 1097-1108. doi: 10.1016/j.jaci.2020.03.029
[13] Gellrich D, Eder K, Högerle C, et al. De novo sensitization during subcutaneous allergen specific immunotherapy-an analysis of 51 cases of SCIT and 33 symptomatically treated controls[J]. Sci Rep, 2020, 10(1): 6048. doi: 10.1038/s41598-020-63087-4
[14] Nittner-Marszalska M, Kopeć A, Foks-Ciekalska A, et al. Monitoring of molecular profiling of allergen-antibody responses in HDM-immunotherapy patients[J]. Hum Vaccin Immunother, 2022, 18(7): 2148815. doi: 10.1080/21645515.2022.2148815
[15] Baron-Bodo V, Batard T, Nguyen H, et al. Absence of IgE neosensitization in house dust mite allergic patients following sublingual immunotherapy[J]. Clin Exp Allergy, 2012, 42(10): 1510-1518. doi: 10.1111/j.1365-2222.2012.04044.x
[16] Lai X, Li J, Xiao X, et al. Specific IgG4 production during house dust mite immunotherapy among age, gender and allergic disease populations[J]. Int Arch Allergy Immunol, 2013, 160(1): 37-46. doi: 10.1159/000339239
[17] Zhao D, Lai X, Tian M, et al. The functional IgE-blocking factor induced by allergen-specific immunotherapy correlates with IgG4 antibodies and a decrease of symptoms in house dust mite-allergic children[J]. Int Arch Allergy Immunol, 2016, 169(2): 113-120. doi: 10.1159/000444391
[18] Chen KW, Zieglmayer P, Zieglmayer R, et al. Selection of house dust mite-allergic patients by molecular diagnosis may enhance success of specific immunotherapy[J]. J Allergy Clin Immunol, 2019, 143(3): 1248-1252.e12. doi: 10.1016/j.jaci.2018.10.048
-




 下载:
下载: