-
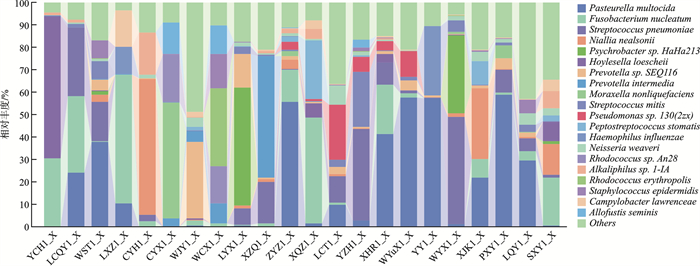
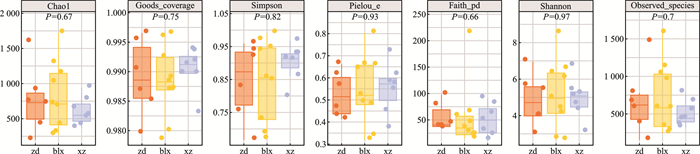
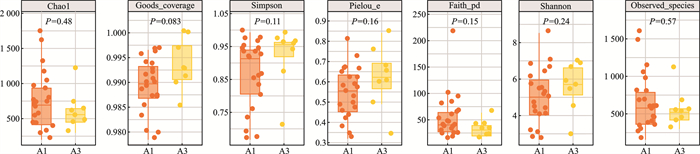
摘要: 目的 通过分析腺样体局部微生物菌群,探讨“病原体储存库”假说。方法 在70°鼻内镜引导下,用无菌拭子采集受试者腺样体隐窝内分泌物,送至实验室提取样本DNA后进行标准细菌16S全长测序分析。结果 在物种水平上测得腺样体隐窝内排前3位的微生物菌群是多杀巴氏杆菌(18.78%),具核梭杆菌(11.42%),肺炎链球菌(9.38%)。结论 腺样体局部微生物菌群具有高度的多样性,包含了来自口腔及消化道的微生物菌群,我们的研究结果也支持了腺样体是“病原体储存库”这一假说。Abstract: Objective To explore the hypothesis of "pathogen storage pool" by analyzing the local microbial community of adenoids.Methods Under the guidance of a 70° nasal endoscope, sterile swabs were used to collect secretions from the adenoid crypts of the subjects. The samples were sent to the laboratory for DNA extraction and standard bacterial 16S full-length sequencing analysis.Results At the species level, the top three microbial communities in adenoid crypts were Bacillus subtilis(18.78%), Fusobacterium pyogenes(11.42%), and Streptococcus pneumoniae(9.38%).Conclusion The local microbial community of adenoids exhibits a high degree of diversity, including microbial communities from the oral cavity and gastrointestinal tract. Our research results support the hypothesis that adenoids act as a " pathogen reservoir".
-
Key words:
- adenoid /
- microbiota /
- 16S rRNA
-

-
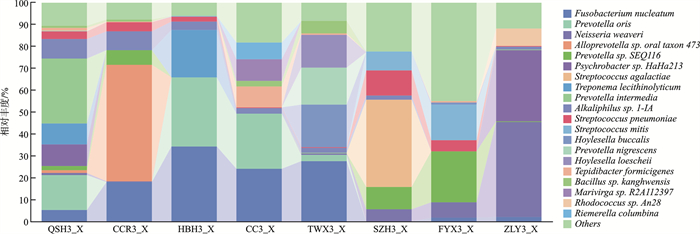
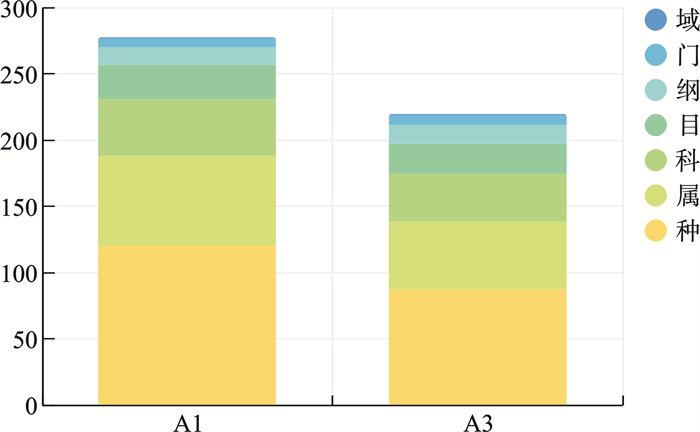
表 1 2组微生物菌群组成
% 菌群名称(物种水平) 病例组(A1) 对照组(A3) Fusobacterium nucleatum(具核梭杆菌) 11.42 14.31 Pasteurella multocida(多杀巴氏杆菌) 18.78 0.51 Streptococcus pneumoniae(肺炎链球菌) 9.38 3.39 Prevotella oris(口腔内普雷沃氏菌) 0.51 9.43 Psychrobacter sp.HaHa213(精神杆菌) 4.10 5.30 Neisseria weaveri(韦氏奈瑟氏球菌) 1.77 7.16 Prevotella sp.SEQ116(普雷沃氏菌) 3.48 5.34 Prevotella intermedia(中间普氏菌) 3.25 3.77 Alloprevotella sp.oral taxon 473 0.10 6.80 Niallia nealsonii(尼尔森氏尼尔菌) 5.44 0.68 Hoylesella loescheii 3.78 1.89 Streptococcus mitis(缓症链球菌) 2.44 3.12 Alkaliphilus sp.1-IA(碱杆菌1-IA) 1.68 3.66 Streptococcus agalactiae(无乳链球菌) 0 4.95 Treponema lecithinolyticum(解卵黄密螺旋体) 0 3.89 Moraxella nonliquefaciens(非液化莫拉氏菌) 2.75 0 Rhodococcus sp.An28(红球菌) 1.71 1.04 Hoylesella buccalis 0.13 2.57 Peptostreptococcus stomatis(胃消化链球菌) 2.04 0.29 Pseudomonas sp.130(2zx)(假单胞菌) 2.32 0 Others(其他未检测细菌) 24.80 21.88 -
[1] Perry M, Whyte A. Immunology of the tonsils[J]. Immunol Today, 1998, 19(9): 414-421. doi: 10.1016/S0167-5699(98)01307-3
[2] Ren TT, Glatt DU, Nguyen TN, et al. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids[J]. Environ Microbiol, 2013, 15(2): 535-547. doi: 10.1111/1462-2920.12000
[3] Hoa M, Tomovic S, Nistico L, et al. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH[J]. Int J Pediatr Otorhinolaryngol, 2009, 73(9): 1242-1248. doi: 10.1016/j.ijporl.2009.05.016
[4] Ngo CC, Massa HM, Thornton RB, et al. Predominant bacteria detected from the middle ear fluid of children experiencing otitis media: a systematic review[J]. PLoS One, 2016, 11(3): e0150949. doi: 10.1371/journal.pone.0150949
[5] Rogers GB, Wesselingh S. Precision respiratory medicine and the microbiome[J]. Lancet Respir Med, 2016, 4(1): 73-82. doi: 10.1016/S2213-2600(15)00476-2
[6] Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens[J]. Nat Rev Immunol, 2013, 13(11): 790-801. doi: 10.1038/nri3535
[7] Subtil J, Rodrigues JC, Reis L, et al. Adenoid bacterial colonization in a paediatric population[J]. Eur Arch Otorhinolaryngol, 2017, 274(4): 1933-1938. doi: 10.1007/s00405-017-4493-z
[8] Xu JH, Dai WJ, Liang Q, et al. The microbiomes of adenoid and middle ear in children with otitis media with effusion and hypertrophy from a tertiary hospital in China[J]. Int J Pediatr Otorhinolaryngol, 2020, 134: 110058. doi: 10.1016/j.ijporl.2020.110058
[9] Kim KS, Min HJ. Correlations between the adenotonsillar microbiome and clinical characteristics of pediatric patients with snoring[J]. Clin Exp Otorhinolaryngol, 2021, 14(3): 295-302. doi: 10.21053/ceo.2020.01634
[10] Swidsinski A, Göktas O, Bessler C, et al. Spatial organisation of microbiota in quiescent adenoiditis and tonsillitis[J]. J Clin Pathol, 2007, 60(3): 253-260. doi: 10.1136/jcp.2006.037309
[11] Cao W, Sun Y, Zhao N, et al. Characteristics of the bacterial microbiota in the upper respiratory tract of children[J]. Eur Arch Otorhinolaryngol, 2022, 279(2): 1081-1089. doi: 10.1007/s00405-021-07013-y
[12] Huang CC, Chang TH, Lee CY, et al. Tissue microbiota in nasopharyngeal adenoid and its association with pneumococcal carriage[J]. Microb Pathog, 2021, 157: 104999. doi: 10.1016/j.micpath.2021.104999
[13] 徐禛, 韩春华, 党志红, 等. 311例腺样体肥大合并分泌性中耳炎患者鼻咽部菌群特征及耐药性分析[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(5): 428-431. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2021.05.010
[14] Peng Z, Wang XR, Zhou R, et al. Pasteurella multocida: genotypes and genomics[J]. Microbiol Mol Biol Rev, 2019, 83(4): e00014-19.
[15] Boyce JD, Harper M, Wilkie IW, et al. Pasteurella[M]//InGyles CL, Prescott JF, Songer JG, et al, Pathogenesis ofbacterial infections in animals. 4th ed. Wiley-Blackwell, Ames, 2010: 325-346.
[16] Brennan CA, Garrett WS. Fusobacterium nucleatum-symbiont, opportunist and oncobacterium[J]. Nat Rev Microbiol, 2019, 17(3): 156-166. doi: 10.1038/s41579-018-0129-6
[17] Engevik MA, Danhof HA, Ruan W, et al. Fusobacterium nucleatum secretes outer membrane vesicles and promotes intestinal inflammation[J]. mBio, 2021, 12(2): e02706-2720. http://www.xueshufan.com/publication/3134865079
[18] Wu X, Wang W, Fang LC, et al. Is Helicobacter pylori colonization associated with chronic tonsillitis?-A meta-analysis and systematic review[J]. Am J Otolaryngol, 2022, 43(5): 103515. doi: 10.1016/j.amjoto.2022.103515
-

| 引用本文: | 袁洛花, 刘海兵, 李文丽, 等. 16S rRNA基因测序下探索腺样体局部微生物菌群[J]. 临床耳鼻咽喉头颈外科杂志, 2025, 39(1): 51-56. doi: 10.13201/j.issn.2096-7993.2025.01.011 |
| Citation: | YUAN Luohua, LIU Haibing, LI Wenli, et al. Exploring local microbial communities in adenoids through 16S rRNA gene sequencing[J]. J Clin Otorhinolaryngol Head Neck Surg, 2025, 39(1): 51-56. doi: 10.13201/j.issn.2096-7993.2025.01.011 |
- Figure 1.
- Figure 2.
- Figure 3.
- Figure 4.
- Figure 5.



 下载:
下载: