The role and the molecular mechanism of abietic acid in the proliferation, invasion and migration of cisplatin-resistant nasopharyngeal carcinoma cells
-
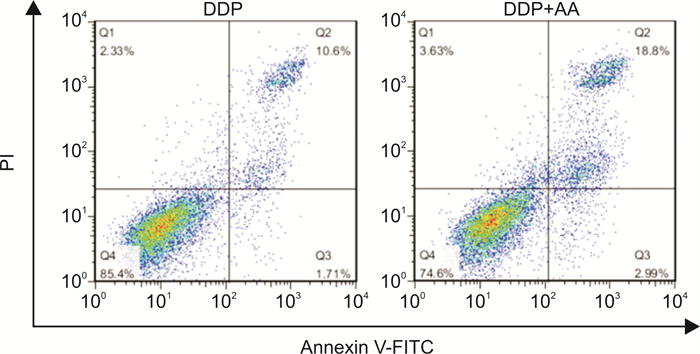
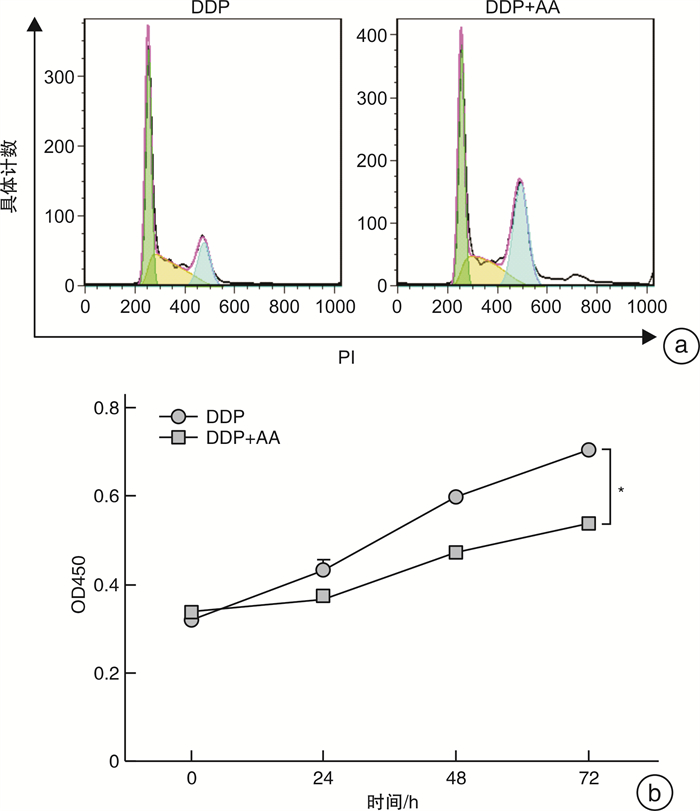
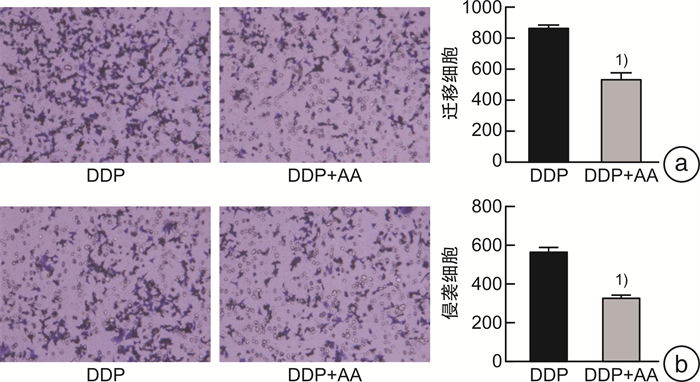
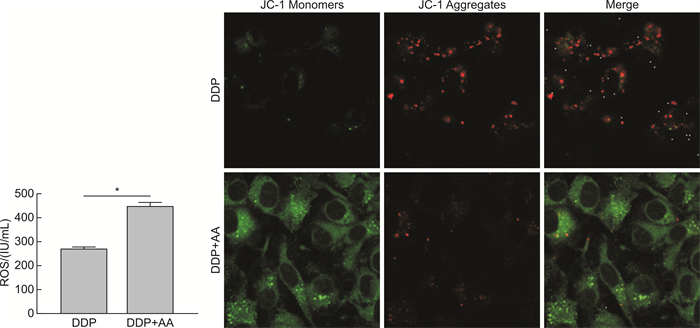
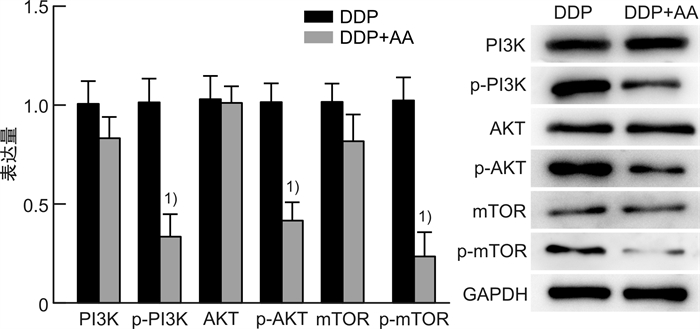
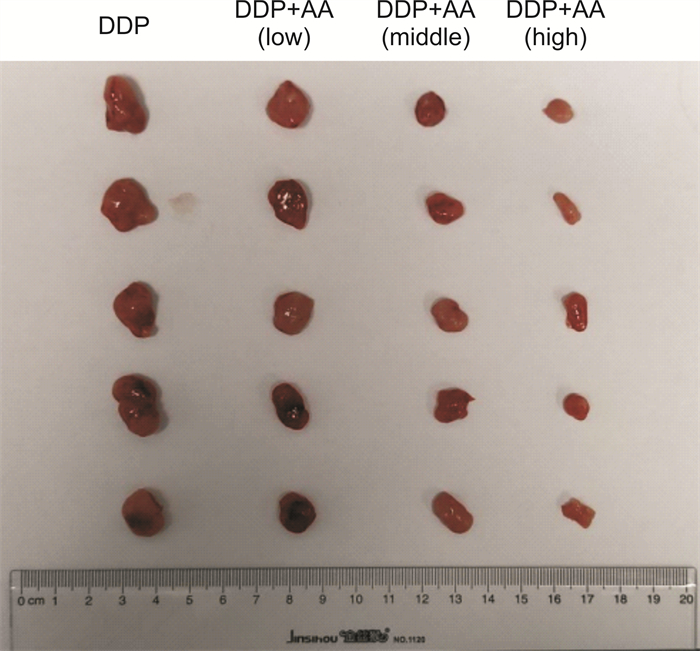
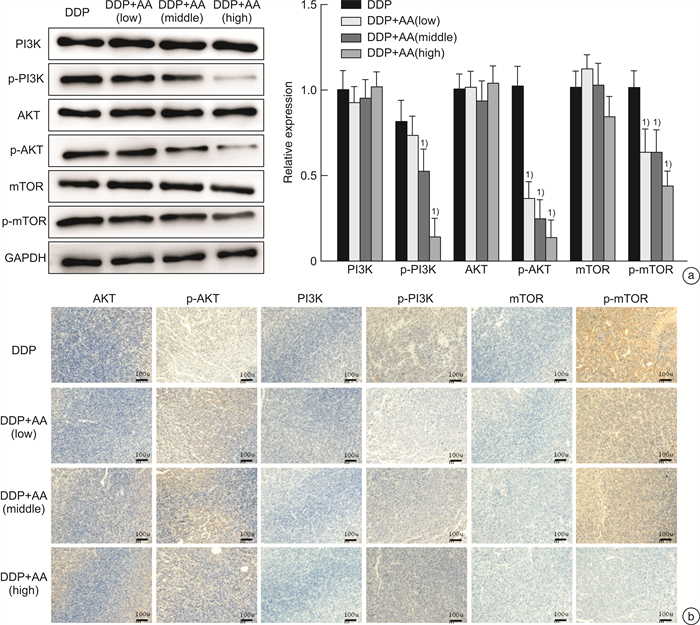
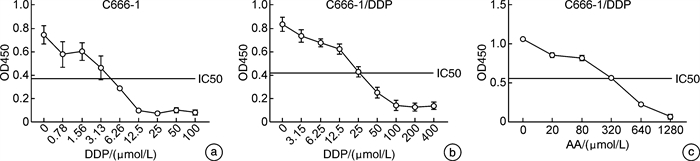
摘要: 目的 探讨枞酸在顺铂耐药鼻咽癌细胞增殖、侵袭、迁移的作用及分子机制。方法 ① 药物浓度递增法构建顺铂耐药鼻咽癌C666/DDP细胞株。②枞酸对C666/DDP细胞增殖、侵袭及迁移的影响研究:CCK-8法、活性氧(ROS)及线粒体膜电位(MMP)水平检测及裸鼠皮下成瘤实验检测枞酸对C666/DDP细胞体外、体内增殖及凋亡能力的影响;Transwell法检测细胞侵袭和迁移能力。③Western blot及IHC法检测PI3K/AKT/mTOR通路相关蛋白的表达情况。结果 ① 顺铂对C666-1细胞毒性IC50约为25 μmol/L。RI=25 μmol/L/4 μmol/L=6.25,获得耐药性,C666-1-DDP耐药株构建成功。②枞酸可促进C666/DDP细胞凋亡,抑制其增殖,且表现为G2/M期阻滞;Transwell显示枞酸抑制C666/DDP细胞迁移及侵袭能力,提高C666/DDP细胞ROS水平并使MMP降低。③动物实验显示枞酸在体内呈浓度梯度抑制顺铂耐药鼻咽癌的增殖,抑制PI3K/AKT/mTOR信号通路相关蛋白的表达。结论 枞酸可抑制顺铂耐药鼻咽癌细胞增殖、侵袭及迁移,其机制与抑制PI3K/AKT/mTOR信号通路有关。Abstract: Objective To investigate the effects and molecular mechanisms of abietic acid in the cell proliferation, invasion and migration of cisplatin-resistant nasopharyngeal carcinoma cells.Methods ① Cisplatin-resistant C666/DDP cell line was constructed by increasing drug concentration method. ②The effects of abietic acid on proliferation, invasion and migration of C666/DDP cells were investigated by CCK-8 method, reactive oxygen species(ROS) and mitochondrial membrane potential(MMP) level assay and subcutaneous tumorigenesis assay in nude mice to detect the effects of abietic acid on proliferation and apoptosis of C666/DDP cells in vitro and in vivo. The effect of abietic acid on the proliferation and apoptosis of C666/DDP cells in vitro and in vivo was measured by Transwell assay. ③Western blot and IHC method to detect the expression of PI3K/AKT/mTOR pathway related proteins.Results ① The IC50 of cisplatin cytotoxicity to C666-1 was about 25 μmol/L. RI=25 μmol/L /4 μmol/L=6.25, resistance was obtained, and the C666-1-DDP resistant strain was successfully constructed. ②Abietic acid promoted apoptosis and inhibited proliferation of C666/DDP cells, and showed G2/M phase block; transwell showed that abietic acid inhibited C666/DDP cell migration and invasion, increased ROS level of C666/DDP cells and decreased MMP. Transwell showed that abietic acid inhibited the migration and invasion ability of C666/DDP cells, increased the ROS level of C666/DDP cells and decreased MMP. ③Animal experiments showed that abietic acid inhibited the proliferation of cisplatin-resistant nasopharyngeal carcinoma in vivo in a concentration gradient and suppressed the expression of PI3K/AKT/mTOR signaling pathway-related proteins.Conclusion Abietic acid inhibits proliferation, invasion and migration of cisplatin-resistant nasopharyngeal carcinoma cells by a mechanism related to inhibition of PI3K/AKT/mTOR signaling pathway.
-
Key words:
- abietic acid /
- nasopharyngeal carcinoma /
- cisplatin resistance /
- molecular mechanism
-

-
[1] Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660
[2] Chen YP, Chan A, Le QT, et al. Nasopharyngeal carcinoma[J]. Lancet, 2019, 394(10192): 64-80. doi: 10.1016/S0140-6736(19)30956-0
[3] Xiang T, Lin YX, Ma W, et al. Vasculogenic mimicry formation in EBV-associated epithelial malignancies[J]. Nat Commun, 2018, 9(1): 5009. doi: 10.1038/s41467-018-07308-5
[4] Zhong L, Dong D, Fang X, et al. A deep learning-based radiomic nomogram for prognosis and treatment decision in advanced nasopharyngeal carcinoma: A multicentre study[J]. EBioMedicine, 2021, 70: 103522. doi: 10.1016/j.ebiom.2021.103522
[5] Tang LL, Chen YP, Chen CB, et al. The Chinese Society of Clinical Oncology(CSCO)clinical guidelines for the diagnosis and treatment of nasopharyngeal carcinoma[J]. Cancer Commun(Lond), 2021, 41(11): 1195-1227.
[6] Lee A, Ng WT, Chan J, et al. Management of locally recurrent nasopharyngeal carcinoma[J]. Cancer Treat Rev, 2019, 79: 101890. doi: 10.1016/j.ctrv.2019.101890
[7] Long Z, Wang W, Liu W, et al. Trend of nasopharyngeal carcinoma mortality and years of life lost in China and its provinces from 2005 to 2020[J]. Int J Cancer, 2022, 151(5): 684-691. doi: 10.1002/ijc.33998
[8] Ghosh S. Cisplatin: The first metal based anticancer drug[J]. Bioorg Chem, 2019, 88: 102925. doi: 10.1016/j.bioorg.2019.102925
[9] Guan S, Wei J, Huang L, et al. Chemotherapy and chemo-resistance in nasopharyngeal carcinoma[J]. Eur J Med Chem, 2020, 207: 112758. doi: 10.1016/j.ejmech.2020.112758
[10] Isah T. Stress and defense responses in plant secondary metabolites production[J]. Biol Res, 2019, 52(1): 39. doi: 10.1186/s40659-019-0246-3
[11] Khan AW, Farooq M, Haseeb M, et al. Role of Plant-Derived Active Constituents in Cancer Treatment and Their Mechanisms of Action[J]. Cells, 2022, 11(8): 1326. doi: 10.3390/cells11081326
[12] Sarwar MS, Zhang HJ, Tsang SW. Perspectives of Plant Natural Products in Inhibition of Cancer Invasion and Metastasis by Regulating Multiple Signaling Pathways[J]. Curr Med Chem, 2018, 25(38): 5057-5087.
[13] Shin SA, Moon SY, Kim WY, et al. Structure-Based Classification and Anti-Cancer Effects of Plant Metabolites[J]. Int J Mol Sci, 2018, 19(9): 2651. doi: 10.3390/ijms19092651
[14] Buommino E, Vollaro A, Nocera FP, et al. Synergistic Effect of Abietic Acid with Oxacillin against Methicillin-Resistant Staphylococcus pseudintermedius[J]. Antibiotics(Basel), 2021, 10(1): 80.
[15] Tretyakova EV, Ma X, Kazakova OB, et al. Abietic, maleopimaric and quinopimaric dipeptide Ugi-4CR derivatives and their potency against influenza A and SARS-CoV-2[J]. Nat Prod Res, 2023, 37(12): 1954-1960. doi: 10.1080/14786419.2022.2112040
[16] Li XQ, Chen Y, Dai GC, et al. Abietic acid ameliorates psoriasis-like inflammation and modulates gut microbiota in mice[J]. J Ethnopharmacol, 2021, 272: 113934. doi: 10.1016/j.jep.2021.113934
[17] Sierra JA, Gilchrist K, Tabares-Guevara JH, et al. Semisynthetic Abietic and Dehydroabietic Acid Derivatives and Triptoquinone Epimers Interfere with LPS-Triggered Activation of Dendritic Cells[J]. Molecules, 2022, 27(19): 6684. doi: 10.3390/molecules27196684
[18] Park JY, Lee YK, Lee DS, et al. Abietic acid isolated from pine resin(Resina Pini)enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice[J]. J Ethnopharmacol, 2017, 203: 279-287. doi: 10.1016/j.jep.2017.03.055
[19] Liu X, Chen W, Liu Q, et al. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKβ/NF-κB signaling[J]. Onco Targets Ther, 2019, 12: 4825-4837. doi: 10.2147/OTT.S199161
[20] Haffez H, Osman S, Ebrahim HY, et al. Growth Inhibition and Apoptotic Effect of Pine Extract and Abietic Acid on MCF-7 Breast Cancer Cells via Alteration of Multiple Gene Expressions Using In Vitro Approach[J]. Molecules, 2022, 27(1): 293. doi: 10.3390/molecules27010293
[21] Xu Y, Tong Y, Lei Z, et al. Abietic acid induces ferroptosis via the activation of the HO-1 pathway in bladder cancer cells[J]. Biomed Pharmacother, 2023, 158: 114154. doi: 10.1016/j.biopha.2022.114154
[22] Hsieh YS, Yang SF, Hsieh YH, et al. Erratum: "The Inhibitory Effect of Abietic Acid on Melanoma Cancer Metastasis and Invasiveness In Vitro and In Vivo"[J]. Am J Chin Med, 2017, 45(2): 403. doi: 10.1142/S0192415X1792001X
[23] Yoshida N, Takada T, Yamamura Y, et al. Inhibitory effects of terpenoids on multidrug resistance-associated protein 2-and breast cancer resistance protein-mediated transport[J]. Drug Metab Dispos, 2008, 36(7): 1206-1211. doi: 10.1124/dmd.107.019513
[24] Miao X, Deng Z, Wang S, et al. IAP-1 promoted cisplatin resistance in nasopharyngeal carcinoma via inhibition of caspase-3-mediated apoptosis[J]. Am J Cancer Res, 2021, 11(3): 640-667.
[25] Vasudevan HN, Yom SS. Nasopharyngeal Carcinoma and Its Association with Epstein-Barr Virus[J]. Hematol Oncol Clin North Am, 2021, 35(5): 963-971. doi: 10.1016/j.hoc.2021.05.007
[26] Prawira A, Oosting SF, Chen TW, et al. Systemic therapies for recurrent or metastatic nasopharyngeal carcinoma: a systematic review[J]. Br J Cancer, 2017, 117(12): 1743-1752. doi: 10.1038/bjc.2017.357
[27] Vallejo MJ, Salazar L, Grijalva M. Oxidative Stress Modulation and ROS-Mediated Toxicity in Cancer: A Review on In Vitro Models for Plant-Derived Compounds[J]. Oxid Med Cell Longev, 2017, 2017: 4586068.
[28] Dharmaraja AT. Role of Reactive Oxygen Species(ROS)in Therapeutics and Drug Resistance in Cancer and Bacteria[J]. J Med Chem, 2017, 60(8): 3221-3240. doi: 10.1021/acs.jmedchem.6b01243
[29] Wu MF, Huang YH, Chiu LY, et al. Curcumin Induces Apoptosis of Chemoresistant Lung Cancer Cells via ROS-Regulated p38 MAPK Phosphorylation[J]. Int J Mol Sci, 2022, 23(15): 8248. doi: 10.3390/ijms23158248
[30] Huang Z, Gan S, Zhuang X, et al. Artesunate Inhibits the Cell Growth in Colorectal Cancer by Promoting ROS-Dependent Cell Senescence and Autophagy[J]. Cells, 2022, 11(16): 2472. doi: 10.3390/cells11162472
[31] Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer[J]. Front Oncol, 2014, 4: 64.
[32] Li HL, Deng NH, He XS, et al. Small biomarkers with massive impacts: PI3K/AKT/mTOR signalling and microRNA crosstalk regulate nasopharyngeal carcinoma[J]. Biomark Res, 2022, 10(1): 52. doi: 10.1186/s40364-022-00397-x
[33] Liu T, Sun Q, Li Q, et al. Dual PI3K/mTOR inhibitors, GSK2126458 and PKI-587, suppress tumor progression and increase radiosensitivity in nasopharyngeal carcinoma[J]. Mol Cancer Ther, 2015, 14(2): 429-439. doi: 10.1158/1535-7163.MCT-14-0548
-




 下载:
下载: