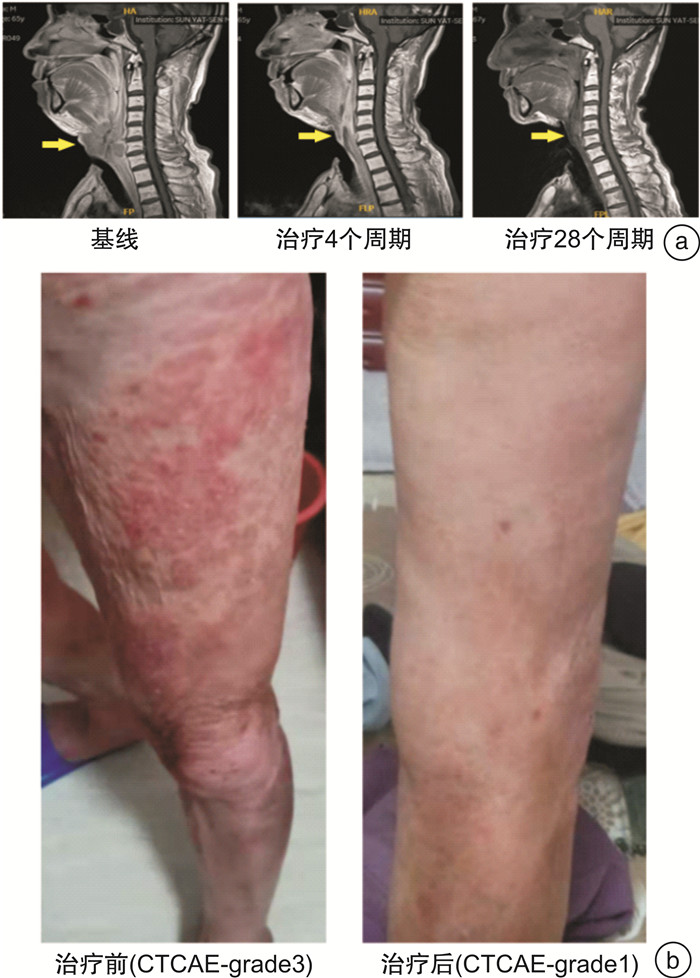
Tislelizumab monotherapy for the treatment of recurrent/metastatic head and neck squamous cell carcinoma
-
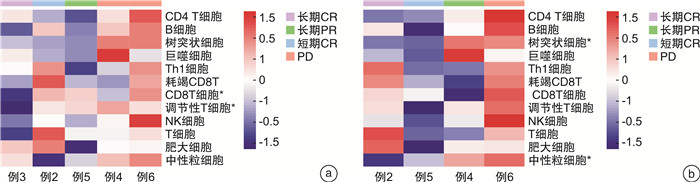
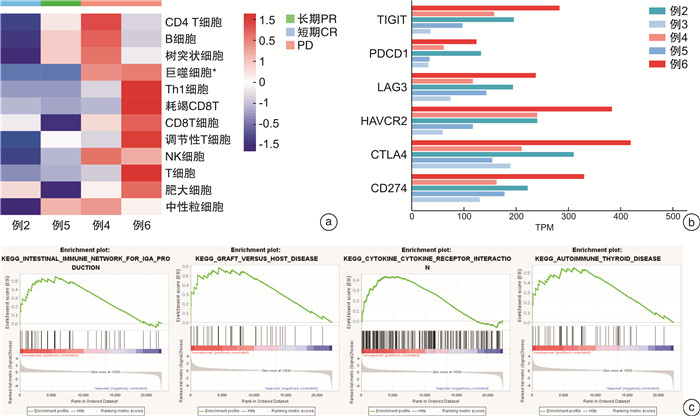
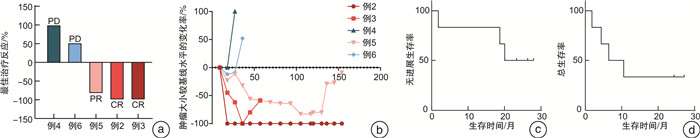
摘要: 目的 评估替雷利珠单抗在复发/转移头颈鳞状细胞癌患者中的安全性和有效性。方法 回顾性分析2018年1月—2020年6月在本院接受替雷利珠单抗单药治疗的复发/转移头颈鳞状细胞癌患者6例,收集性别、年龄、TNM分期、疗效、不良反应等信息。所有患者均来自RATIONALE 102研究,主要研究终点为客观缓解率,其他终点包括无进展生存期和总生存期等。对患者肿瘤组织进行了肿瘤免疫相关基因测序以及转录组测序分析,利用生物信息学方法进行免疫细胞富集及信号通路分析。所有分析均采用R语言4.1.0版、SPSS Statistics 24.0软件和GraphPad Prism 8完成。结果 截至2020年5月31日,中位随访时间为26.35个月。替雷利珠单抗治疗的客观缓解率为50.0%,中位无进展生存期为6.44个月,估计中位生存期为20.07个月。3级以上不良反应发生率66.7%,包括低钠血症、低钾血症、高钙血症等。免疫治疗敏感患者的巨噬细胞、Treg和中性粒细胞相关基因表达较高,产生IgA的肠道免疫网络、移植物抗宿主病、自身免疫性甲状腺疾病等信号通路明显激活。结论 替雷利珠单抗治疗复发或转移头颈鳞状细胞癌患者具有可控和可耐受的安全性,治疗反应与免疫细胞浸润及免疫相关信号通路的激活有关。Abstract: Objective The aim of this retrospective study is to evaluate the safety and efficacy of tislelizumab in patients with recurrent/metastatic head and neck squamous cell carcinoma.Methods Six patients with recurrent/metastatic head and neck squamous cell carcinoma who received tislelizumab monotherapy in our hospital from 2018 to 2020 were retrospectively analyzed. The information of sex, age, TNM stage, efficacy, and adverse reactions were collected. All patients were recruited from the RATIONALE 102 study. The primary end point was the objective response rate, and other end points included progression-free survival and overall survival. We performed tumor immune-related gene sequencing and transcriptome sequencing analysis on the tumor tissues of the patient, and used bioinformatics methods to enrich immune cells and analyze signaling pathways. All analyses were performed using R 4.1. 0 software, SPSS Statistics 24.0 software and GraphPad Prism 8.Results As of May 31, 2020, the median follow-up time was 26.35 months. The objective response rate with tislelizumab was 50.0%, the median progression-free survival was 6.44 months, and the estimated median survival was 20.07 months. The incidence of grade 3 or higher adverse reactions was 66.7%, including hyponatremia, hypokalemia, hypercalcemia, etc. The expression of macrophage, Treg and neutrophil-related genes are higher in immune-sensitive patients, and the signaling pathways of the intestinal immune network for IgA production, graft versus host disease and autoimmune thyroid disease are significantly activated.Conclusion Tislelizumab was found to be controllable and tolerable in patients with recurrent/metastatic head and neck squamous cell carcinoma. The response to tislelizumab is related to immune cell infiltration and activation of immune-related signaling pathways.
-
Key words:
- head and neck squamous cell carcinoma /
- tislelizumab /
- immunotherapy
-

-
表 1 人口统计学和临床特征
例序 年龄/岁 性别 原发灶部位 TNM分期 CPS评分 用药周期数 最佳总体疗效 研究终点结局 1 64 男 下咽 T4N2cM1 10 3 NA 死亡 2 54 男 喉 T4N2M0 10 16 CR PD 3 65 男 喉 T4N0M0 40 34 CR NA 4 37 女 下咽 T0N2M0 20 7 SD PD 5 51 男 喉 T2N2M0 30 38 PR NA 6 45 女 口腔 T4N0M0 20 11 SD PD NA:无法评估;SD:疾病稳定。 表 2 替雷利珠单抗治疗期间的不良反应
n=6,例(%) TRAEs Ⅰ级 Ⅱ级 Ⅲ级 IrAEs 体重减轻 3(50.00) 1(17.00) 0 否 低钾血症 1(17.00) 0 2(33.00) 否 低钠血症 2(33.00) 0 1(17.00) 否 咳痰 0 3(50.00) 0 否 咳嗽 1(17.00) 2(33.00) 0 否 颈痛 1(17.00) 2(33.00) 0 否 甲状腺功能减退 1(17.00) 2(33.00) 0 是 贫血 2(33.00) 1(17.00) 0 否 低蛋白血症 3(50.00) 0 0 否 头晕 3(50.00) 0 0 否 肺部感染 0 0 2(33.00) 否 吞咽困难 0 1(17.00) 1(17.00) 否 体重增加 1(17.00) 1(17.00) 0 否 发烧 1(17.00) 1(17.00) 0 否 高糖血症 1(17.00) 1(17.00) 0 否 皮肤瘙痒 2(33.00) 0 0 是 低氯血症 2(33.00) 0 0 否 高钙血症 0 0 1(17.00) 否 亚临床甲状腺功能减退症 0 1(17.00) 0 是 皮疹 0 1(17.00) 0 是 白细胞增多 0 1(17.00) 0 否 胸闷 0 1(17.00) 0 否 口腔溃疡 0 1(17.00) 0 是 流鼻涕 0 1(17.00) 0 否 血尿 1(17.00) 0 0 否 呕吐 1(17.00) 0 0 否 关节酸痛 1(17.00) 0 0 否 低钙血症 1(17.00) 0 0 否 尿道感染 1(17.00) 0 0 否 高尿酸血症 1(17.00) 0 0 否 霍纳综合征 1(17.00) 0 0 否 牙痛 1(17.00) 0 0 否 口干 1(17.00) 0 0 否 恶心 1(17.00) 0 0 否 -
[1] Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment[J]. Mayo Clin Proc, 2008, 83(4): 489-501. doi: 10.4065/83.4.489
[2] Chow LQM. Head and Neck Cancer[J]. N Engl J Med, 2020, 382(1): 60-72. doi: 10.1056/NEJMra1715715
[3] 宋攀, 颜晓晴, 姜燕慧, 等. PD-1/L1抑制剂在头颈部鳞状细胞癌治疗中的应用进展[J]. 临床耳鼻咽喉头颈外科杂志, 2022, 36(04): 315-320. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.2096-7993.2022.04.017
[4] 严晓菊, 徐开伦, 张欣欣. 头颈鳞状细胞癌的免疫治疗[J]. 临床耳鼻咽喉头颈外科杂志, 2017, 31(13): 1050-1056. https://lceh.whuhzzs.com/article/doi/10.13201/j.issn.1001-1781.2017.13.024
[5] Pfister D, Spencer S, Adelstein D, et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology[J]. Journal of the National Comprehensive Cancer Network: JNCCN, 2020, 18(7): 873-898.
[6] Borel C, Jung AC, Burgy M. Immunotherapy Breakthroughs in the Treatment of Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma[J]. Cancers(Basel), 2020, 12(9): 2691.
[7] Zhang T, Song X, Xu L, et al. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions[J]. Cancer Immunol Immunother, 2018, 67(7): 1079-1090. doi: 10.1007/s00262-018-2160-x
[8] Lee A, Keam SJ. Tislelizumab: First Approval[J]. Drugs, 2020, 80(6): 617-624. doi: 10.1007/s40265-020-01286-z
[9] Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline(version 1.1)[J]. Eur J Cancer, 2009, 45(2): 228-47. doi: 10.1016/j.ejca.2008.10.026
[10] Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer[J]. Arch Pathol Lab Med, 2019, 143(3): 330-337.
[11] Wang Z, Zhao J, Ma Z, et al. A Phase 2 Study of Tislelizumab in Combination With Platinum-Based Chemotherapy as First-line Treatment for Advanced Lung Cancer in Chinese Patients[J]. Lung Cancer, 2020, (147): 259-268.
[12] Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck(KEYNOTE-048): a randomised, open-label, phase 3 study[J]. Lancet, 2019, 394(10212): 1915-1928.
[13] Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer[J]. J Clin Oncol, 2015, 33(29): 3293-304.
[14] Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck(KEYNOTE-012): an open-label, multicentre, phase 1b trial[J]. Lancet Oncol, 2016, 17(7): 956-965.
[15] Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression[J]. Oral Oncol, 2018, 81: 45-51.
[16] Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck[J]. N Engl J Med, 2016, 375(19): 1856-1867.
[17] Kiyota N, Hasegawa Y, Takahashi S, et al. A randomized, open-label, Phase Ⅲ clinical trial of nivolumab vs. therapy of investigator's choice in recurrent squamous cell carcinoma of the head and neck: A subanalysis of Asian patients versus the global population in checkmate 141[J]. Oral Oncol, 2017, Oct(73): 138-146.
[18] Tahara M, Muro K, Hasegawa Y, et al. Pembrolizumab in Asia-Pacific patients with advanced head and neck squamous cell carcinoma: Analyses from KEYNOTE-012[J]. Cancer Sci, 2018, 109(3): 771-776.
[19] Desai J, Deva S, Lee JS, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody, in solid tumors[J]. J Immunother Cancer, 2020, 8(1): e000453.
[20] Reck M, Rodriguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater[J]. J Clin Oncol, 2019, 37(7): 537-546.
[21] Emancipator K, Huang L, Aurora-Garg D, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer[J]. Mod Pathol, 2021, 34(3): 532-541.
[22] Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase Ⅱ Study[J]. J Clin Oncol, 2017, 35(14): 1542-1549.
[23] Cohen EEW, Bell RB, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck(HNSCC)[J]. J Immunother Cancer., 2019, 7(1): 184.
[24] Meng X, Huang Z, Teng F, et al. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy[J]. Cancer Treat Rev, 2015, 41(10): 868-876.
[25] Sanchez-Canteli M, Granda-Díaz R, Del Rio-Ibisate N, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas[J]. Cancer Immunol Immunother, 2020, 69(10): 2089-2100.
[26] Zhou Z, Mu D, Zhang D, et al. PD-L1 in Combination with CD8(+)TIL and HIF-1alpha are Promising Prognosis Predictors of Head and Neck Squamous Cell Carcinoma[J]. Cancer Manag Res, 2020, 23(12): 13233-13239.
[27] Drennan S, Stafford ND, Greenman J, et al. Increased frequency and suppressive activity of CD127(low/-)regulatory T cells in the peripheral circulation of patients with head and neck squamous cell carcinoma are associated with advanced stage and nodal involvement[J]. Immunology, 2013, 140(3): 335-343.
[28] Troiano G, Caponio VCA, Adipietro I, et al. Prognostic significance of CD68(+)and CD163(+)tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis[J]. Oral Oncol, 2019, Jun(93): 66-75.
[29] Tran Janco JM, Lamichhane P, Karyampudi L, et al. Tumor-infiltrating dendritic cells in cancer pathogenesis[J]. J Immunol, 2015, 194(7): 2985-2991.
[30] Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemot herapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer(I Mpassion031): a randomised, double-blind, phase 3 trial[J]. Lancet, 2020, 396(10257): 1090-1100.
[31] Ferrarotto R, Bell D, Rubin ML, et al. Impact of Neoadjuvant Durvalumab with or without Tremelimumab on CD8(+)Tumor Lymphocyte Density, Safety, and Efficacy in Patients with Oropharynx Cancer: CIAO Trial Results[J]. Clin Cancer Res, 2020, 26(13): 3211-3219.
[32] Shen L, Guo J, Zhang Q, et al. Tislelizumab in Chinese patients with advanced solid tumors: an open-label, non-comparative, phase 1/2 study[J]. J Immunother Cancer, 2020, 8(1): e000437.
[33] Sznol M, Ferrucci PF, Hogg D, et al. Pooled Analysis Safety Profile of Nivolumab and Ipilimumab Combination Therapy in Patients With Advanced Melanoma[J]. J Clin Oncol, 2017, 35(34): 3815-3822.
[34] Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors[J]. Nat Rev Dis Primers, 2020, 6(1): 38.
[35] Foster CC, Couey MA, Kochanny SE, et al. Immune-related adverse events are associated with improved response, progression-free survival, and overall survival for patients with head and neck cancer receiving immune checkpoint inhibitors[J]. Cancer, 2021, 127(24): 4565-4573.
-




 下载:
下载: