-
摘要: 过敏原鼻激发试验(nasal provocation test,NPT)是指在标准、有控制的条件下将过敏原直接作用于鼻腔黏膜,以观察其是否可激发出变应性鼻炎(allergic rhinitis,AR)的主要症状,再现上呼吸道对受控条件下自然暴露的过敏原的反应,且是目前唯一可用的确认鼻腔对过敏原反应性的测试。它在研究AR的机制和评估新型抗过敏治疗反应方面具有非常重要的价值。NPT在临床实践中的作用越来越大,尤其在局部AR的鉴别、职业性AR的诊断、明确过敏原组分、AR治疗效果评价和进行过敏原免疫治疗的患者选择等方面作用更大、更广。本文综述了NPT在变应性和非变应性鼻炎等诊断中的应用,并对临床实践中使用NPT评估鼻腔反应的适应证、禁忌证、优点和局限性等进行介绍。Abstract: The allergen nasal provocation testing(NPT), in which allergens are applied directly to the nasal mucosa under standard and controlled conditions to provoke the main symptoms of allergic rhinitis(AR), reproduces the response of the upper respiratory tract to natural exposure to allergens under controlled conditions and is the only test currently available to confirm nasal reactivity to allergens. It is invaluable in studying the mechanisms of AR and in assessing the response to novel anti-allergic treatments. The test may play an increasingly important role in clinical practice, especially in the identification of local AR, the diagnosis of occupational AR, the clarification of the composition of allergens, the assessment of the efficacy of AR treatment and the selection of candidates undergoing allergen immunotherapy. This article reviewed the application of NPT in the diagnosis of allergic and non-allergic rhinitis, and also introduces the indications, contraindications, advantages and limitations of NPT in evaluating nasal response.
-
Key words:
- nasal provocation test /
- allergen /
- allergic rhinitis /
- local allergic rhinitis /
- non-allergic rhinitis
-

-
表 1 关于NPT适应证与禁忌证的建议
适应证 过敏原的鉴定 科学目的 ①研究过敏反应的机制和各种制剂对过敏反应的影响;
②确定临床研究中重现鼻腔症状所需的过敏原最大剂量阈值。临床诊断 ①确定AIT的指征,确定直接导致患者症状的过敏原,因此确定疫苗的成分,监测脱敏和药物治疗的效果;
②AR、LAR和职业AR(可能伴有哮喘);
③食物过敏的诊断;
④支气管哮喘的替代诊断;
⑤阿司匹林加重性呼吸系统疾病(aspirin-exacerbated respiratory disease,AERD)的辅助诊断;
⑥对难以接受疾病后果的患者进行确诊,如不养宠物或换工作。禁忌证 绝对禁忌证 ①怀孕或哺乳;
②患有不稳定和严重的全身性疾病患者,如不稳定缺血性心脏病、严重循环衰竭、恶性肿瘤和自身免疫性疾病(如韦格纳病,丘格-施特劳斯综合征)等活动期或加重期;
③正在接受全身抗肿瘤的免疫治疗;
④服用β-肾上腺素抑制药物或血管紧张素转换酶抑制剂、使用肾上腺素抢救有禁忌证的患者;
⑤无法控制的哮喘或慢性阻塞性肺疾病(FEV1 < 70%);
⑥无应对过敏性休克所需的药物和设备。相对禁忌证 ①年龄 < 5岁;
②没有标准化的过敏原提取物;
③鼻腔解剖异常、慢性疾病:鼻畸形、后鼻孔闭锁、鼻中隔穿孔、严重鼻中隔偏曲、伴或不伴鼻息肉的慢性鼻窦炎、萎缩性鼻炎;
④身体其他部位的急性过敏反应、极高的致敏程度;
⑤多次暴露于化学刺激物或吸烟史。阶段性禁忌证 ①患者处于有症状期,如Lebel评分>3分;
②在NPT前1周接种疫苗、使用过抗组胺药或血管收缩药等,1个月内使用过鼻用糖皮质激素,3个月内使用过全身糖皮质激素;
③呼吸道严重的细菌或病毒感染(4周内);
④鼻腔、鼻窦手术后2~3个月内;
⑤在检查前24~48 h内饮酒或吸烟、吃辛辣食物和(或)喝咖啡。表 2 NPT症状评分标准
症状 评分标准 打喷嚏 0分:0~2个 1分:3~5个 2分:>5个 鼻分泌物 0分:无 1分:少量(≤1 mL) 2分:多量(>1 mL) 鼻外症状 0分:无 1分:腭痒、眼痒、耳痒 2分:结膜炎、球结膜水肿、荨麻疹、咳嗽、呼吸困难 表 3 2018年EAACI关于NPT阳性标准的建议
评估方法 强阳性(+++) 中等强度阳性(++) 主观评估 VAS评分 ≥5.5分 ≥2.3分 Lebel评分 增加≥5.0分 增加≥3.0分 Linder评分 增加≥5.0分 增加≥3.0分 TNSS 增加≥5.0分 增加≥3.0分 客观评估 PNIF 下降≥40% 下降≥20% 鼻声反射 距前鼻孔2 cm的横截面积下降≥40% 容积总和下降≥27% 主动经前鼻测压法 在150 Pa下鼻流量减少≥40% 在150 Pa下鼻流量减少≥20% 四相鼻阻力测试 对数曲线有效阻力增加≥40% 对数曲线有效阻力增加≥20% -
[1] Izquierdo-Domínguez A, Bobolea I, Doña I, et al. Statement of the Spanish Society of Allergology and Clinical Immunology on Provocation Tests With Aspirin/Nonsteroidal Anti-inflammatory Drugs[J]. J Investig Allergol Clin Immunol, 2020, 30(1): 1-13. doi: 10.18176/jiaci.0449
[2] Skaarup SH, Schmid JM, Skjold T, et al. Intralymphatic immunotherapy improves grass pollen allergic rhinoconjunctivitis: A 3-year randomized placebo-controlled trial[J]. J Allergy Clin Immunol, 2021, 147(3): 1011-1019. doi: 10.1016/j.jaci.2020.07.002
[3] Jungewelter S, Airaksinen L, Pesonen M. Occupational rhinitis, asthma, and contact urticaria from IgE-mediated allergy to pork[J]. Am J Ind Med, 2019, 62(1): 80-84. doi: 10.1002/ajim.22921
[4] Sánchez A, Cardona R, Munera M, et al. Nasal Provocation Test with Cat and Dog Extracts: Results according to Molecular Components[J]. Pulm Med, 2020, 2020: 6365314. http://pubmed.ncbi.nlm.nih.gov/32047667/
[5] Pepper AN, Ledford DK. Nasal and ocular challenges[J]. J Allergy Clin Immunol, 2018, 141(5): 1570-1577. doi: 10.1016/j.jaci.2017.11.066
[6] Al-Ahmad M, Jusufovic E, Arifhodzic N, et al. Validity of Skin Prick Test to Bermuda Grass in a desert environment[J]. Acta Biomed, 2021, 92(4): e2021218. http://pubmed.ncbi.nlm.nih.gov/34487076/
[7] Al-Ahmad M, Jusufovic E, Arifhodzic N, et al. Sensitization to Cat: When Is Nasal Challenge Needed?[J]. Int Arch Allergy Immunol, 2019, 179(2): 108-113. doi: 10.1159/000496835
[8] Eguiluz-Gracia I, Testera-Montes A, González M, et al. Safety and reproducibility of nasal allergen challenge[J]. Allergy, 2019, 74(6): 1125-1134. doi: 10.1111/all.13728
[9] Englhard AS, Holzer M, Eder K, et al. How reliable is anamnestic data in predicting the clinical relevance of house dust mite sensitization?[J]. Eur Arch Otorhinolaryngol, 2022, 279(2): 801-810. doi: 10.1007/s00405-021-06862-x
[10] Joo SH, Hyun KJ, Kim YH. Korean Modification of the Nasal Provocation Test With House Dust Mite Antigen Following the EAACI Guidelines[J]. Clin Exp Otorhinolaryngol, 2021, 14(4): 382-389. doi: 10.21053/ceo.2020.00563
[11] Kim YH. Appropriate Antigen Concentrations and Timing of a Nasal Provocation Test[J]. Yonsei Med J, 2021, 62(8): 750-757. doi: 10.3349/ymj.2021.62.8.750
[12] Krzych-Fałta E. The conjunctival allergen provocation test and the nasal allergen provocation test as specific alternatives to the oral food challenge[J]. Postepy Dermatol Alergol, 2022, 39(2): 245-250. doi: 10.5114/ada.2021.105362
[13] Tantilipikorn P, Siriboonkoom P, Sookrung N, et al. Prevalence of local allergic rhinitis to Dermatophagoides pteronyssinus in chronic rhinitis with negative skin prick test[J]. Asian Pac J Allergy Immunol, 2021, 39(2): 111-116. http://www.ncbi.nlm.nih.gov/pubmed/30903999
[14] 肖浩, 孟娟, 张虹婷, 等. 鼻腔黏膜激发试验的临床应用及研究进展[J]. 中国耳鼻咽喉头颈外科, 2019, 26(2): 112-116. doi: 10.16066/j.1672-7002.2019.02.016
[15] 娄鸿飞, 黄嫣然, 张罗, 等. 干冷空气鼻激发试验诊断特发性鼻炎的研究[J]. 临床耳鼻咽喉头颈外科杂志, 2020, 34(8): 673-677. doi: 10.13201/j.issn.2096-7993.2020.08.001
[16] Huang Y, Lou H, Wang C, et al. Cold dry air provocation is a reliable diagnostic tool in nonallergic rhinitis[J]. Rhinology, 2019, 57(3): 225-230. http://www.ncbi.nlm.nih.gov/pubmed/30964471
[17] Traiyan S, Manuyakorn W, Kanchongkittiphon W, et al. Skin Prick Test Versus Phadiatop as a Tool for Diagnosis of Allergic Rhinitis in Children[J]. Am J Rhinol Allergy, 2021, 35(1): 98-106. doi: 10.1177/1945892420938300
[18] Thamrongsak C, Chirdkiatgumchai V, Jotikasthira W, et al. Improvement of inattentive and hyperactive symptoms after real-life rhinitis treatment in school-aged children[J]. Int J Pediatr Otorhinolaryngol, 2022, 157: 111138. doi: 10.1016/j.ijporl.2022.111138
[19] 中华耳鼻咽喉头颈外科杂志编辑委员会鼻科组, 中华医学会耳鼻咽喉头颈外科学分会鼻科学组. 中国变应性鼻炎诊断和治疗指南(2022年, 修订版)[J]. 中华耳鼻咽喉头颈外科杂志, 2022, 57(2): 106-129. doi: 10.3760/cma.j.cn115330-20211228-00828
[20] Augé J, Vent J, Agache I, et al. EAACI Position paper on the standardization of nasal allergen challenges[J]. Allergy, 2018, 73(8): 1597-1608. doi: 10.1111/all.13416
[21] 王洪田, 于睿莉, 安云芳, 等. 变应原鼻腔激发试验中国专家共识(2022, 北京)[J]. 中国眼耳鼻喉科杂志, 2023, 23(1): 1-10. https://www.cnki.com.cn/Article/CJFDTOTAL-YRBH202301001.htm
[22] Krzych-Fałta E, Piekarska B, Sybilski A, et al. The Safety of Nasal Allergen Challenge Test Assessed in Lower Airways[J]. Iran J Allergy Asthma Immunol, 2015, 14(6): 581-588. http://www.researchgate.net/profile/Adam_Sybilski/publication/286567931_The_Safety_of_Nasal_Allergen_Challenge_Test_Assessed_in_Lower_Airways/links/566bd64108ae1a797e3bb808.pdf
[23] Yang WH, Kelly S, Haya L, et al. Cat allergen exposure in a naturalistic exposure chamber: A prospective observational study in cat-allergic subjects[J]. Clin Exp Allergy, 2022, 52(2): 265-275. doi: 10.1111/cea.14087
[24] Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper[J]. World Allergy Organ J, 2020, 13(2): 100080. doi: 10.1016/j.waojou.2019.100080
[25] Pfaar O, Calderon MA, Andrews CP, et al. Allergen exposure chambers: harmonizing current concepts and projecting the needs for the future-an EAACI Position Paper[J]. Allergy, 2017, 72(7): 1035-1042. doi: 10.1111/all.13133
[26] Hossenbaccus L, Ellis AK. The use of nasal allergen vs allergen exposure chambers to evaluate allergen immunotherapy[J]. Expert Rev Clin Immunol, 2021, 17(5): 461-470. doi: 10.1080/1744666X.2021.1905523
[27] Larson D, Patel P, Salapatek AM, et al. Nasal allergen challenge and environmental exposure chamber challenge: A randomized trial comparing clinical and biological responses to cat allergen[J]. J Allergy Clin Immunol, 2020, 145(6): 1585-1597. doi: 10.1016/j.jaci.2020.02.024
[28] Fuller JC, Bernstein CH, Levesque PA, et al. Peak Nasal Inspiratory Flow as an Objective Measure of Nasal Obstruction and Functional Septorhinoplasty Outcomes[J]. JAMA Facial Plast Surg, 2018, 20(2): 175-176. doi: 10.1001/jamafacial.2017.1775
[29] Wandalsen GF, Mendes AI, Matsumoto F, et al. Acoustic Rhinometry in Nasal Provocation Tests in Children and Adolescents[J]. J Investig Allergol Clin Immunol, 2016, 26(3): 156-160. doi: 10.18176/jiaci.0036
[30] Welkoborsky HJ, Rose-Diekmann C, Vor der Holte AP, et al. Clinical parameters influencing the results of anterior rhinomanometry in children[J]. Eur Arch Otorhinolaryngol, 2022, 279(8): 3963-3972. doi: 10.1007/s00405-021-07218-1
[31] Wojas O, Szczêsnowicz-Dąbrowska P, Krzych-Fałta E, et al. Changes in the cross-sections of the nasal cavity assessed by acoustic rhinometry in the study population as a guideline for attempts to standardize nasal provocation tests[J]. Postepy Dermatol Alergol, 2022, 39(2): 347-352. doi: 10.5114/ada.2021.105361
[32] Vogt K, Wernecke KD, Behrbohm H, et al. Four-phase rhinomanometry: a multicentric retrospective analysis of 36, 563 clinical measurements[J]. Eur Arch Otorhinolaryngol, 2016, 273(5): 1185-1198. doi: 10.1007/s00405-015-3723-5
[33] Agache I, Bilò M, Braunstahl GJ, et al. In vivo diagnosis of allergic diseases--allergen provocation tests[J]. Allergy, 2015, 70(4): 355-365. doi: 10.1111/all.12586
[34] Rudman Spergel AK, Sever ML, Johnson J, et al. Development of nasal allergen challenge with cockroach in children with asthma[J]. Pediatr Allergy Immunol, 2021, 32(5): 971-979. doi: 10.1111/pai.13480
[35] Tenn MW, Rawls M, Ellis AK. Nasal challenges in allergen immunotherapy trials[J]. Curr Opin Allergy Clin Immunol, 2018, 18(6): 489-494.
[36] Huang R, Qin R, Hu Q, et al. Effect of Dermatophagoides pteronyssinus Immunotherapy on Upper and Lower Airway Eosinophilic Inflammatory Response to Nasal Allergen Challenge[J]. Allergy Asthma Immunol Res, 2020, 12(5): 844-858. doi: 10.4168/aair.2020.12.5.844
[37] Nieto A, Mazón Á, Nieto M, et al. First-in-human phase 2 trial with mite allergoids coupled to mannan in subcutaneous and sublingual immunotherapy[J]. Allergy, 2022, 77(10): 3096-3107.
[38] Prieto A, Rondón C, Eguiluz-Gracia I, et al. Systematic evaluation of allergic phenotypes of rhinitis in children and adolescents[J]. Pediatr Allergy Immunol, 2021, 32(5): 953-962. doi: 10.1111/pai.13474
[39] Campo P, Eguiluz-Gracia I, Plaza-Serón MC, et al. Bronchial asthma triggered by house dust mites in patients with local allergic rhinitis[J]. Allergy, 2019, 74(8): 1502-1510. http://www.onacademic.com/detail/journal_1000042300572399_3d74.html
[40] Beken B, Eguiluz-Gracia I, Yazıcıoǧlu M, et al. Local allergic rhinitis: a pediatric perspective[J]. Turk J Pediatr, 2020, 62(5): 701-710. doi: 10.24953/turkjped.2020.05.001
[41] Bozek A, Winterstein J, Galuszka B, et al. Different Development Forms of Local Allergic Rhinitis towards Birch[J]. Biomed Res Int, 2020, 2020: 3408561.
[42] Bozek A, Scierski W, Ignasiak B, et al. The prevalence and characteristics of local allergic rhinitis in Poland[J]. Rhinology, 2019, 57(3): 213-218.
[43] Meng Y, Wang Y, Lou H, et al. Specific immunoglobulin E in nasal secretions for the diagnosis of local allergic rhinitis[J]. Rhinology, 2019, 57(4): 313-320.
[44] Tsilochristou O, Kyriakakou M, Manolaraki I, et al. Detection of local allergic rhinitis in children with chronic, difficult-to-treat, non-allergic rhinitis using multiple nasal provocation tests[J]. Pediatr Allergy Immunol, 2019, 30(3): 296-304. http://www.ncbi.nlm.nih.gov/pubmed/30685887
[45] Ronsmans S, Steelant B, Backaert W, et al. Diagnostic approach to occupational rhinitis: the role of nasal provocation tests[J]. Curr Opin Allergy Clin Immunol, 2020, 20(2): 122-130. http://pubmed.ncbi.nlm.nih.gov/31833858/
[46] Vandenplas O, Hox V, Bernstein D. Occupational Rhinitis[J]. J Allergy Clin Immunol Pract, 2020, 8(10): 3311-3321.
[47] Jungewelter S, Airaksinen L, Pesonen M. Occupational buckwheat allergy as a cause of allergic rhinitis, asthma, contact urticaria and anaphylaxis-An emerging problem in food-handling occupations?[J]. Am J Ind Med, 2020, 63(11): 1047-1053. http://pubmed.ncbi.nlm.nih.gov/32944967/
[48] Jungewelter S, Suomela S, Airaksinen L. Occupational IgE-mediated psyllium allergy in contemporary gluten-free and vegan baking: A case of allergic rhinitis[J]. Am J Ind Med, 2021, 64(5): 431-434. http://pubmed.ncbi.nlm.nih.gov/33651455/
[49] Kotz S, Pechtold L, Jörres RA, et al. Occupational rhinitis[J]. Allergol Select, 2021, 5: 51-56.
[50] Fischl A, Eckrich J, Passlack V, et al. Comparison of bronchial and nasal allergen provocation in children and adolescents with bronchial asthma and house dust mite sensitization[J]. Pediatr Allergy Immunol, 2020, 31(2): 143-149. http://pubmed.ncbi.nlm.nih.gov/31660641/
[51] Ramírez-Jiménez F, Vázquez-Corona A, Sánchez-de la Vega Reynoso P, et al. Effect of LTRA in L-ASA Challenge for Aspirin-Exacerbated Respiratory Disease Diagnosis[J]. J Allergy Clin Immunol Pract, 2021, 9(4): 1554-1561. http://pubmed.ncbi.nlm.nih.gov/33160093/
[52] Krzych-Fałta E, Wojas O, Samel-Kowalik P, et al. Nasal mucosal reactivity assessment via a double-blind placebo-controlled food challenge with cow's milk allergens[J]. Allergy Asthma Clin Immunol, 2022, 18(1): 59. doi: 10.1186/s13223-022-00700-3
-

| 引用本文: | 韩婕, 陆美萍, 程雷. 过敏原鼻激发试验研究进展及临床应用[J]. 临床耳鼻咽喉头颈外科杂志, 2023, 37(6): 415-422. doi: 10.13201/j.issn.2096-7993.2023.06.003 |
| Citation: | HAN Jie, LU Meiping, CHENG Lei. Research progress and clinical application of allergen nasal provocation test[J]. J Clin Otorhinolaryngol Head Neck Surg, 2023, 37(6): 415-422. doi: 10.13201/j.issn.2096-7993.2023.06.003 |
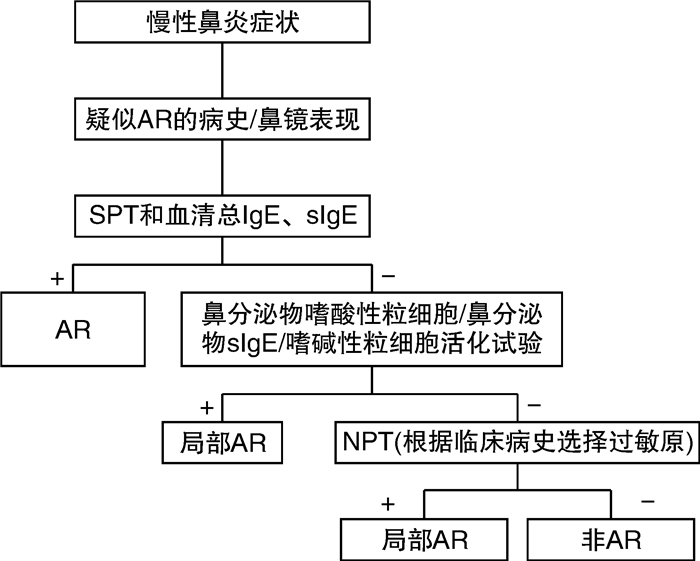
- Figure 1.



 下载:
下载: