The efficacy of OM85-BV in the treatment of recurrent upper respiratory tract infection with adenoid hypertrophy and the preliminary exploration of potential therapeutic mechanism
-
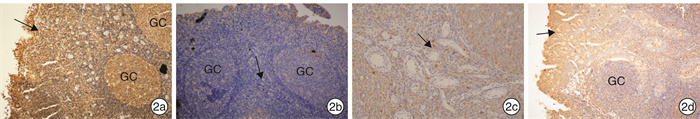
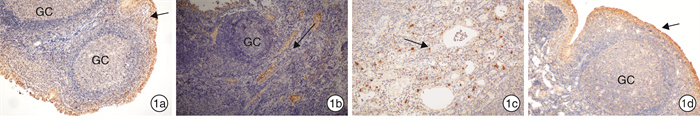
摘要: 目的 观察OM85-BV治疗反复上呼吸道感染伴腺样体肥大的疗效并对其治疗机制进行初步探讨。方法 448例反复上呼吸道感染伴腺样体肥大的患儿,根据自主选择是否接受OM85-BV治疗分为对照组和观察组,对照组326例采用常规药物治疗,观察组122例采用OM85-BV治疗+常规药物治疗。2组患儿治疗周期均为12周,在治疗的不同时段(入组时、治疗6周、治疗12周),根据OSA-18改良中文版对患儿睡眠呼吸阻塞症状进行评分,比较2组患儿症状评分结果和治疗有效率。2组经过12周药物治疗仍无效者接受腺样体切除术。比较2组中接受手术治疗的患儿术前血清IL-2、IL-4、IL-6、IL-10、TNF、IFN-γ细胞因子含量和IgE含量,血清CD3、CD4、CD8在淋巴细胞中的比例及CD4/CD8比值情况。比较2组中接受腺样体切除术患儿腺样体HBD-2、IFN-γ、IL-4、IL-6细胞因子含量、表达水平差异及分布特征。结果 经过12周的治疗,观察组总有效率明显高于对照组,反复上呼吸道感染伴腺样体肥大儿童的睡眠呼吸阻塞症状的改善程度也明显高于对照组。观察组中血清IFN-γ明显高于对照组,血清IL-2、IL-4、IL-6、IL-10、TNF、IgE与对照组相比无明显差异。观察组中血清CD3、CD4、CD8、CD4/CD8与对照组相比无明显差异。观察组腺样体HBD-2明显高于对照组,IL-4、IFN-γ明显低于对照组,IL-6与对照组相比无明显差异。结论 OM85-BV可明显改善反复上呼吸道感染伴腺样体肥大患儿的睡眠障碍,却不能改变患儿体内免疫淋巴细胞的水平。OM85-BV既可提高机体的Th1免疫防御反应,又可增强机体对病原的抵抗力,诱导腺样体局部HBD-2的表达增加,增强局部腺样体对微生物抵抗力,使定植于腺样体的细菌减少,减弱腺样体局部炎症反应。Abstract: Objective To observe the efficacy of OM85-BV in the treatment of recurrent upper respiratory tract infection with adenoid hypertrophy and to explore its possible mechanism.Method Four hundred and forty-eight children with recurrent upper respiratory tract infection and adenoid hypertrophy were collected. Three hundred and twenty-six patients in the control group were treated with conventional drugs, and one hundred and twenty-two patients in the observation group were treated with OM85-BV+conventional drugs, and the treatment lasted 12 weeks. The sleep obstructive symptoms of adenoid hypertrophy were scored according to OSA-18 before and after the treatment respectively(0, 6, 12 weeks). The symptoms scores and effective rate of treatment between the study and the control group were compared. The patients in the control group and the observation group who were unresponsive to drug treatment received surgery after 12 weeks of drug treatment. The levels of serum IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ and IgE, the ratio of serum CD3, CD4, CD8 in lymphocytes and the ratio of CD4/CD8 were compared between the study and the control group before operation. The levels of HBD-2, IFN-γ, IL-4, IL-6 cytokines in the adenoid were compared between the control group and the observation group. The expression and distribution of adenoid HBD-2, IFN-γ, IL-4, IL-6 were compared between the control group and the observation group.Result After 12 weeks of treatment, the total effective rate of the observation group was significantly higher than that of the control group, and the improvement of sleep respiratory obstruction symptoms of children with recurrent upper respiratory tract infection and adenoid hypertrophy was also much better than that of the control group. The serum IFN-γ of the observation group was significantly higher than that of the control group, and there was no significant difference in serum IL-2, IL-4, IL-6, IL-10, TNF, IgE between the observation group and the control group. There was no significant difference in serum CD3, CD4, CD8 and CD4/CD8 between the observation group and the control group. In the observation group, the adenoid HBD-2 was significantly higher but IL-4, IFN-γ were significantly lower than that in the control group, and IL-6 had no significant difference compared with the control group.Conclusion OM85-BV can significantly improve the sleep apnea symptoms but can not rise the level of immune lymphocytes in children with adenoid hypertrophy and recurrent upper respiratory tract infection.OM85-BV can improve the Th1 immune response, enhancing the ability of human body to fight against pathogens and induce the release of HBD-2, increasing the resistance to microorganisms, reducing the bacteria aggregation, weakening the local inflammatory response in adenoids.
-
Key words:
- adenoid hypertrophy /
- OM85-BV /
- upper respiratory infections
-

-
表 1 2组研究对象的一般资料比较
组别 例数 男 女 年龄/岁 腺样体A/N 入组时第1次问卷评分 入组时IgE水平/ (pg·mL-1) 对照组 326 190 136 5.42±2.04 0.65±0.11 21.43±12.365 95.0±10.4 观察组 122 71 51 5.81±2.15 0.67±0.23 23.44±13.182 94.0±13.2 表 2 2组治疗效果比较
例(%) 组别 例数 显效 有效 无效 总有效 对照组 326 85(26.1) 104(31.9) 137(42.0) 189(58.0) 观察组 122 47(38.5) 55(45.1) 20(16.4) 102(83.6) -
[1] Berger M, Geng B, Cameron DW, et al. Primary immune deficiency diseases as unrecognized causes of chronic respiratory disease[J]. Respir Med, 2017, 132: 181-188. doi: 10.1016/j.rmed.2017.10.016
[2] Zupin L, Celsi F, Bresciani M, et al. Human beta defensin-1 is involved in the susceptibility to adeno-tonsillar hypertrophy[J]. Int J Pediatr Otorhinolaryngol, 2018, 107: 135-139. doi: 10.1016/j.ijporl.2018.01.041
[3] Triantafillou V, Workman AD, Patel NN, et al. Broncho-Vaxom®(OM-85 BV)soluble components stimulate sinonasal innate immunity[J]. Int Forum Allergy Rhinol, 2019, 9(4): 370-377. doi: 10.1002/alr.22276
[4] Esposito S, Bianchini S, Bosis S, et al. A randomized, placebo-controlled, double-blinded, single-centre, phase IV trial to assess the efficacy and safety of OM-85 in children suffering from recurrent respiratory tract infections[J]. J Transl Med, 2019, 17(1): 284-284. doi: 10.1186/s12967-019-2040-y
[5] 张波. 反复呼吸道感染的临床概念和处理原则[J]. 世界最新医学信息文摘, 2017, 17(62): 194-194. https://www.cnki.com.cn/Article/CJFDTOTAL-WMIA201762163.htm
[6] Acar M, Kankilic ES, Koksal AO, et al. Methodsof the diagnosis of adenoid hypertrophy for physicians: adenoid-nasopharynx ratio[J]. J Craniofac Surg, 2014, 25(5): e438-440. doi: 10.1097/SCS.0000000000000952
[7] 仇书要, 刘大波, 钟建文. 2018法国耳鼻咽喉头颈外科学会指南解读: 不同治疗方案在儿童OSAHS治疗中的地位[J]. 临床耳鼻咽喉头颈外科杂志, 2020, 34(2): 97-100. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH202002001.htm
[8] 周永, 莫贤海, 宾翔, 等. OSA-18调查表法对儿童阻塞性睡眠呼吸障碍患儿生活质量评估的临床意义[J]. 中国耳鼻咽喉颅底外科杂志, 2014, 20(6): 502-506. https://www.cnki.com.cn/Article/CJFDTOTAL-ZEBY201406010.htm
[9] Yan XH, Zhao Y, Wang J, et al. Associations among sleep symptoms, physical examination, and polysomnographic findings in children with obstructive sleep apnea[J]. Eur Arch Otorhinolaryngol, 2020, 277(2): 623-630. doi: 10.1007/s00405-019-05719-8
[10] Koatz AM, Coe NA, Cicerán A, et al. Clinical and Immunological Benefits of OM-85 Bacterial Lysate in Patients with Allergic Rhinitis, Asthma, and COPD and Recurrent Respiratory Infections[J]. Lung, 2016, 194(4): 687-697. doi: 10.1007/s00408-016-9880-5
[11] Bodemer C, Guillet G, Cambazard F, et al. Adjuvant treatment with the bacterial lysate(OM-85) improves management of atopic dermatitis: A randomized study[J]. PLoS One, 2017, 12(3): e0161555. doi: 10.1371/journal.pone.0161555
[12] Rossi GA, Bessler W, Ballarini S, et al. Evidence that a primary anti-viral stimulation of the immune response by OM-85 reducessusceptibility to a secondary respiratory bacterial infection in mice[J]. Ital J Pediatr, 2018, 44(1): 112-112. doi: 10.1186/s13052-018-0569-7
[13] Luan H1, Zhang Q, Wang L. OM85-BV induced the productions of IL-1β, IL-6, and TNF-α via TLR4-and TLR2-mediated ERK1/2/NF-κB pathway in RAW264.7 cells[J]. J Interferon Cytokine Res, 2014, 34(7): 526-536. doi: 10.1089/jir.2013.0077
[14] Coviello S, Wimmenauer V, Polack FP, et al. Bacterial lysates improve the protective antibody response against respiratory viruses through Toll-like receptor 4[J]. Hum Vaccin Immunother, 2014, 10(10): 2896-2902. doi: 10.4161/hv.29784
[15] Parola C, Salogni L, Vaira X, et al. eCollection 2013. Selective activation of human dendritic cells by OM-85 through a NF-kB and MAPK dependent pathway[J]. PLoS One, 2013, 8(12): e82867. doi: 10.1371/journal.pone.0082867
[16] Dang AT, Pasquali C, Ludigs K, et al. OM-85 is an immunomodulator of interferon-β production and inflammasome activity[J]. Sci Rep, 2017, 7: 43844. doi: 10.1038/srep43844
[17] Emeryk A, Bartkowiak-Emeryk M, Raus Z, et al. Mechanical bacterial lysate administration prevents exacerbation in allergic asthmatic children-The EOLIA study[J]. Pediatr Allergy Immunol, 2018, 29(4): 394-401. doi: 10.1111/pai.12894
[18] Han L, Zheng CP, Sun YQ, et al. A bacterial extract of OM-85 Broncho-Vaxom prevents allergic rhinitis in mice[J]. Am J Rhinol Allergy, 2014, 28(2): 110-116. doi: 10.2500/ajra.2013.27.4021
[19] Liu C, Huang R, Yao R, et al. The Immunotherapeutic Role of Bacterial Lysates in a Mouse Model of Asthma[J]. Lung, 2017, 195(5): 563-569. doi: 10.1007/s00408-017-0003-8
[20] Esposito S, Soto-Martinez ME, Feleszko W, et al. Nonspecific immunomodulators for recurrent respiratory tract infections, wheezing and asthma in children: a systematic review of mechanistic and clinical evidence[J]. Curr Opin Allergy Clin Immunol, 2018, 18(3): 198-209. doi: 10.1097/ACI.0000000000000433
[21] Lu Y, Li Y, Xu L, et al. Bacterial Lysate Increases the Percentage of Natural Killer T Cells in Peripheral Blood and Alleviates Asthma in Children[J]. Pharmacology, 2015, 95(3-4): 139-144. . doi: 10.1159/000377683
[22] Scott NM, Lauzon-Joset JF, Jones AC, et al. Protection against maternal infection-associated fetal growth restriction: proof-of-concept with a microbial-derived immunomodulator[J]. Mucosal Immunol, 2017, 10(3): 789-801. doi: 10.1038/mi.2016.85
[23] Berber A, Del-Rio-Navarro BE. Cost-effectiveness analysis of OM-85 vs placebo in the prevention of acute respiratory tractinfections(ARTIs)in children that attend day-care centers[J]. Health Econ Rev, 2019, 9(1): 12-12. doi: 10.1186/s13561-019-0230-1
[24] 张丰珍, 王桂香, 许志飞, 等. 儿童重度OSAHS睡眠结构及相关因素分析[J]. 临床耳鼻咽喉头颈外科杂志, 2019, 33(5): 441-446. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH201905015.htm
[25] Donnarumma G, Paoletti I, Fusco A, et al. β-Defensins: Work in Progress[J]. Adv Exp Med Biol, 2016, 901: 59-76.
[26] 廖嘉仪, 张涛. 细菌溶解产物对支气管哮喘合并反复呼吸道感染患儿血清β防御素1及免疫球蛋白的影响[J]. 中国当代儿科杂志, 2014, 16(5): 508-512. https://www.cnki.com.cn/Article/CJFDTOTAL-DDKZ201405014.htm
[27] Guaní-Guerra E, Negrete-García MC, Montes-Vizuet R, et al. Human β-defensin-2 induction in nasal mucosa after administration of bacterial lysates[J]. Arch Med Res, 2011, 42(3): 189-194. doi: 10.1016/j.arcmed.2011.04.003
-




 下载:
下载: