Effect of sublingual immunotherapy on inflammatory factors and autophagy in patients with allergic rhinitis
-
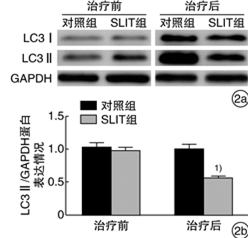
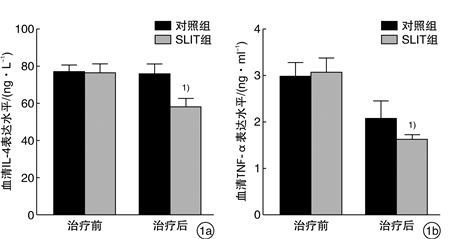
摘要: 目的 观察舌下免疫治疗对变应性鼻炎(AR)患者血清炎症因子IL-4、TNF-α及鼻黏膜自噬相关蛋白LC3水平的影响。方法 将40例AR患者随机分为舌下含服免疫治疗(SLIT)组(n=20)和对照组(n=20),SLIT组采用标准化尘螨疫苗SLIT联合常规药物进行为期2年的干预,对照组采用安慰剂及常规药物治疗。收集2组患者治疗前后的血液标本和下鼻甲黏膜;分析2组患者治疗前后的临床症状、体征和用药评分;ELISA检测治疗前后血清IL-4、TNF-α表达水平;Western blot检测治疗前后自噬相关蛋白LC3的表达情况。结果 SLIT组与对照组治疗前体征、症状、用药评分、年龄、性别、血清IL-4和TNF-α以及LC3表达情况差异无统计学意义(P>0.05)。治疗2年后,SLIT组患者体征、症状评分均较对照组显著改善;血清IL-4、TNF-α表达水平较对照组显著下降;自噬相关蛋白LC3表达情况显著低于对照组,均差异有统计学意义(P < 0.05)。结论 相比单纯药物治疗,SLIT联合常规药物治疗对AR患者的症状改善更加明显,SLIT在一定程度上能减轻AR患者的炎症水平以及自噬相关蛋白的表达。Abstract: Objective To observe the effect of sublingual immunotherapy on the expression of serum inflammatory factors IL-4 and TNF-α as well as autophagy-associated protein LC3 in nasal mucosa in patients with allergic rhinitis(AR).Method Forty patients with AR were randomly divided into SLIT group(n=20) and control group(n=20), the SLIT group received a 2-year intervention with a standardized dust mite vaccine SLIT in combination with conventional drugs, the control group received placebo and conventional drug treatment. Blood samples and inferior turbinate mucosa were collected of both groups before and after the treatment; the clinical symptoms, signs and medication scores of the two groups before and after treatment were analyzed; the expressions of serum IL-4 and TNF-α were detected by ELISA before and after treatment; the expression of autophagy-related protein LC3 was detected by Western blot.Result There were no significant differences in the pre-treatment signs, symptoms, medication scores, age, gender, serum IL-4, TNF-α, and LC3 expression between the SLIT group and the control group(P>0.05). After a 2-year treatment, the symptom scores the of SLIT group were significantly improved compared with the control group; serum levels of IL-4 and TNF-α were significantly decreased in the SLIT group; the expression of autophagy-related protein LC3 in the SLIT group was significantly lower than that in the control group, and the difference was statistically significant(P < 0.05).Conclusion SLIT combined with conventional drug therapy is more effective in improving the symptoms of AR patients than conventional drug therapy. SLIT can reduce the inflammation level and expression of autophagy-related proteins in AR patients to a certain extent.
-
Key words:
- rhinitis, allergic /
- sublingual immunotherapy /
- inflammatory factors /
- autophagy
-

-
表 1 症状评分标准
分级计分/分 喷嚏/个a) 流涕/次b) 鼻塞 鼻痒 0 < 3 0 无症状 无症状 1 3~5 ≤4 有意识吸气时感觉鼻塞 间断鼻痒 2 6~10 5~9 间歇或交互性 蚁行感,可忍受 3 ≥11 ≥10 几乎全天用口呼吸 蚁行感,难忍 a)一次连续喷嚏个数;b)每日擤鼻次数。 表 2 2组患者治疗前后临床症状、体征和用药评分比较
中位数[25分位数~75分位数] 组别 例数 症状评分 体征评分 用药评分 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 对照组 20 8.0(7.0~10.0) 3.0(1.0~5.0) 2.0(2.0~3.0) 1.0(1.0~3.0) 0.0(0.0~0.0) 0.0(0.0~0.0) SLIT组 20 9.0(8.0~10.0) 1.0(1.0~2.0) 2.0(2.0~4.0) 0.0(0.0~0.0) 0.0(0.0~0.0) 0.0(0.0~0.0) P >0.05 < 0.05 >0.05 < 0.05 >0.05 >0.05 -
[1] Seidman M, Gurgel R, Lin S, et al. Clinical practice guideline: allergic rhinitis[J]. Otolaryngol Head Neck Surg, 2015, 152(1 Suppl): S1-43.
[2] Meltzer E, Bukstein D. The economic impact of allergic rhinitis and current guidelines for treatment[J]. Ann Allergy Asthma Immunol, 2011, 106(2 Suppl): S12-16.
[3] Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues[J]. Cell, 2011, 147(4): 728-741. doi: 10.1016/j.cell.2011.10.026
[4] Hammad H, Lambrecht B. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma[J]. Nat Rev Immunol, 2008, 8(3): 193-204. doi: 10.1038/nri2275
[5] Moldoveanu B, Otmishi O, Jani P, et al. Inflammatory mechanisms in the lung[J]. J Inflamm Res, 2009, 2: 1-11.
[6] Holgate S. Pathogenesis of asthma[J]. Clin Exp Allergy, 2008, 38(6): 872-897. doi: 10.1111/j.1365-2222.2008.02971.x
[7] Lambrecht B, Hammad H. The immunology of asthma[J]. Nat Immunol, 2015, 16(1): 45-56. doi: 10.1038/ni.3049
[8] Bradding P, Walls A, Holgate S. The role of the mast cell in the pathophysiology of asthma[J]. J Allergy Clin Immunol, 2006, 117(6): 1277-1284. doi: 10.1016/j.jaci.2006.02.039
[9] Levine B, Mizushima N, Virgin H. Autophagy in immunity and inflammation[J]. Nature, 2011, 469(7330): 323-335. doi: 10.1038/nature09782
[10] Bousquet J, Khaltaev N, Cruz A, et al. Allergic Rhinitis and its Impact on Asthma(ARIA)2008 update(in collaboration with the World Health Organization, GA(2) LEN and AllerGen)[J]. Allergy, 2008, 63 Suppl 86: 8-160.
[11] Mizushima N, Yoshimori T, Levine B. Methodsin mammalian autophagy research[J]. Cell, 2010, 140(3): 313-326. doi: 10.1016/j.cell.2010.01.028
[12] 李静, 李勇. 自噬在变应性鼻炎中的作用[J]. 中华临床免疫和变态反应杂志, 2018, 12(6): 437-640. https://www.cnki.com.cn/Article/CJFDTOTAL-OZHL201806029.htm
[13] Ban G, Pham D, Trinh T, et al. Autophagy mechanisms in sputum and peripheral blood cells of patients with severe asthma: a new therapeutic target[J]. Clin Exp Allergy, 2016, 46(1): 48-59. doi: 10.1111/cea.12585
[14] 余杰情, 罗庆, 熊园平, 等. 变应性鼻炎中自噬相关基因LC3与ECP的表达及意义[J]. 临床耳鼻咽喉头颈外科杂志, 2019, 33(4): 322-325. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH201904009.htm
[15] Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells[J]. Tissue Barriers, 2013, 1(2): E24333. doi: 10.4161/tisb.24333
[16] Lee JK, Lee S, Baek MC, et al. Association between perfluorooctanoic acid exposure and degranulation of mast cells in allergic inflammation[J]. J Appl Toxicol, 2017, 37(5): 554-562. doi: 10.1002/jat.3389
[17] Subramanian N, Bray M. Interleukin 1 releases histamine from human basophils and mast cells in vitro[J]. J Immunol, 1987, 138(1): 271-275.
[18] Minai-Fleminger Y, Levi-Schaffer F. Mast cells and eosinophils: the two key effector cells in allergic inflammation[J]. Inflamm Res, 2009, 58(10): 631-638. doi: 10.1007/s00011-009-0042-6
[19] Bischoff S. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data[J]. Nat Rev Immunol, 2007, 7(2): 93-104. doi: 10.1038/nri2018
[20] Shakoory B, Fitzgerald SM, Lee SA, et al. The role of human mast cell-derived cytokines in eosinophil biology[J]. J Interferon Cytokine Res, 2004, 24(5): 271-281. doi: 10.1089/107999004323065057
[21] Wenzel S. Asthma phenotypes: the evolution from clinical to molecular approaches[J]. Nat Med, 2012, 18(5): 716-725. doi: 10.1038/nm.2678
-




 下载:
下载: