-
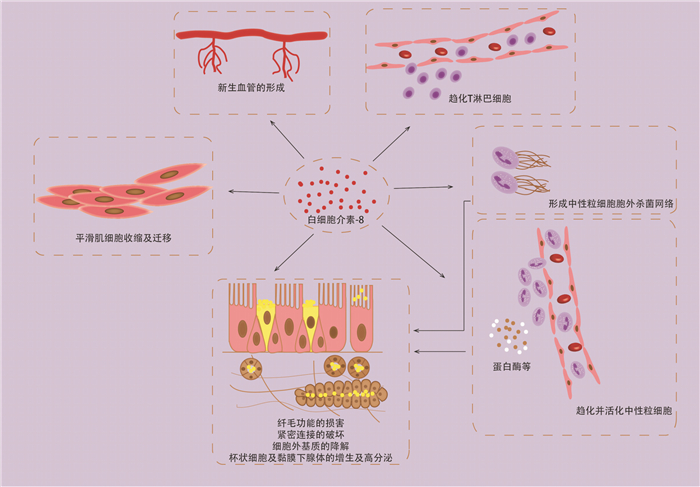
Abstract: Chronic rhinosinusitis(CRS), asthma and chronic obstructive pulmonary disease(COPD) are common chronic airway inflammatory diseases, which seriously affect patients' quality of life and bring heavy economic and social burden. Interleukin(IL) -8 is an important chemokine of neutrophils and other inflammatory cells, which plays an important role in the development of chronic airway inflammation. In this review, the inflammatory mechanisms involved in regulating IL-8 gene expression and the role of IL-8 in different airway chronic inflammation were reviewed.
-
Key words:
- airway inflammation /
- interleukin-8 /
- pathogenesis
-
-
[1] Braunstahl GJ, Overbeek SE, Kleinjan A, et al. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways[J]. J Allergy Clin Immunol, 2001, 107(3): 469-476. doi: 10.1067/mai.2001.113046
[2] Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma(ARIA)guidelines-2016 revision[J]. J Allergy Clin Immunol, 2017, 140(4): 950-958. doi: 10.1016/j.jaci.2017.03.050
[3] Samitas K, Carter A, Kariyawasam HH, et al. Upper and lower airway remodelling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited[J]. Allergy, 2018, 73(5): 993-1002. doi: 10.1111/all.13373
[4] 李伟利, 叶成刚, 胡惠玲, 等. 哮喘与阿司匹林哮喘影响慢性鼻窦炎的临床研究[J]. 临床耳鼻咽喉头颈外科杂志, 2019, 33(7): 635-638. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH201907014.htm
[5] Bergin DA, Hurley K, Mehta A, et al. Airway inflammatory markers in individuals with cystic fibrosis and non-cystic fibrosis bronchiectasis[J]. J Inflamm Res, 2013, 6: 1-11.
[6] Guo C, Sun X, Diao W, et al. Correlation of Clinical Symptoms and Sputum Inflammatory Markers with Air Pollutants in Stable COPD Patients in Beijing Area[J]. Int J Chron Obstruct Pulmon Dis, 2020, 15: 1507-1517. doi: 10.2147/COPD.S254129
[7] Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: A multicenter study in Europe, Asia, and Oceania[J]. J Allergy Clin Immunol, 2016, 138(5): 1344-1353. doi: 10.1016/j.jaci.2016.05.041
[8] Jundi K, Greene CM. Transcription of Interleukin-8: How Altered Regulation Can Affect Cystic Fibrosis Lung Disease[J]. Biomolecules, 2015, 5(3): 1386-1398. doi: 10.3390/biom5031386
[9] Li J, Jiao J, Wang M, et al. Hypomethylation of the IL8 promoter in nasal epithelial cells of patients with chronic rhinosinusitis with nasal polyps[J]. J Allergy Clin Immunol, 2019, 144(4): 993-1003. doi: 10.1016/j.jaci.2019.06.042
[10] Akdis M, Aab A, Altunbulakli C, et al. Interleukins(from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases[J]. J Allergy Clin Immunol, 2016, 138(4): 984-1010. doi: 10.1016/j.jaci.2016.06.033
[11] Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 Axis in Cancer and Inflammatory Diseases[J]. Theranostics, 2017, 7(6): 1543-1588. doi: 10.7150/thno.15625
[12] Liu X, Yin S, Chen Y, et al. LPSinduced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NFkappaB, STAT3 or AP1 activation[J]. Mol Med Rep, 2018, 17(4): 5484-5491.
[13] Anzalone G, Gagliardo R, Bucchieri F, et al. IL-17A induces chromatin remodeling promoting IL-8 release in bronchial epithelial cells: Effect of Tiotropium[J]. Life Sciences, 2016, 152: 107-116. doi: 10.1016/j.lfs.2016.03.031
[14] Bartling TR, Drumm ML. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models[J]. Am J Respir Cell Mol Biol, 2009, 40(1): 58-65. doi: 10.1165/rcmb.2007-0464OC
[15] Hoffmann E, Dittrich-Breiholz O, Holtmann H, et al. Multiple control of interleukin-8 gene expression[J]. J Leukoc Biol, 2002, 72(5): 847-855.
[16] Lee CW, Chung SW, Bae MJ, et al. Peptidoglycan Up-Regulates CXCL8 Expression via Multiple Pathways in Monocytes/Macrophages[J]. Biomol Ther(Seoul), 2015, 23(6): 564-570. doi: 10.4062/biomolther.2015.053
[17] Neuschafer-Rube F, Pathe-Neuschafer-Rube A, Hippenstiel S, et al. PGE2 enhanced TNFalpha-mediated IL-8 induction in monocytic cell lines and PBMC[J]. Cytokine, 2019: 113: 105-116. doi: 10.1016/j.cyto.2018.06.020
[18] Ang Z, Koean RAG, Er JZ, et al. Novel AU-rich proximal UTR sequences(APS)enhance CXCL8 synthesis upon the induction of rpS6 phosphorylation[J]. PLoS Genet, 2019, 15(4): e1008077. doi: 10.1371/journal.pgen.1008077
[19] Najdaghi S, Razi S, Rezaei N. An overview of the role of interleukin-8 in colorectal cancer[J]. Cytokine, 2020, 135: 155205. doi: 10.1016/j.cyto.2020.155205
[20] Hidalgo MA, Carretta MD, Teuber SE, et al. fMLP-Induced IL-8 Release Is Dependent on NADPH Oxidase in Human Neutrophils[J]. J Immunol Res, 2015, 2015: 120348.
[21] Moodie FM, Marwick JA, Anderson CS, et al. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells[J]. FASEB J, 2004, 18(15): 1897-1899. doi: 10.1096/fj.04-1506fje
[22] Yang SR, Chida AS, Bauter MR, et al. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages[J]. Am J Physiol Lung Cell Mol Physiol, 2006, 291(1): L46-57. doi: 10.1152/ajplung.00241.2005
[23] Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020[J]. Rhinology, 2020, 58(Suppl S29): 1-464.
[24] Kim DW, Eun KM, Roh EY, et al. Chronic Rhinosinusitis without Nasal Polyps in Asian Patients Shows Mixed Inflammatory Patterns and Neutrophil-Related Disease Severity[J]. Mediators Inflamm, 2019, 2019: 7138643.
[25] Shen JC, Chen B, Cohen NA. Keratinocyte chemoattractant(interleukin-8) regulation of sinonasal cilia function in a murine model[J]. Int Forum Allergy Rhinol, 2012, 2(1): 75-79. doi: 10.1002/alr.20087
[26] Yoon BN, Choi NG, Lee HS, et al. Induction of interleukin-8 from nasal epithelial cells during bacterial infection: the role of IL-8 for neutrophil recruitment in chronic rhinosinusitis[J]. Mediators Inflamm, 2010, 2010: 813610.
[27] Kao SS, Ramezanpour M, Bassiouni A, et al. The effect of neutrophil serine proteases on human nasal epithelial cell barrier function[J]. Int Forum Allergy Rhinol, 2019, 9(10): 1220-1226. doi: 10.1002/alr.22401
[28] Yan D, Ye Y, Zhang J, et al. Human Neutrophil Elastase Induces MUC5AC Overexpression in Chronic Rhinosinusitis Through miR-146a[J]. Am J Rhinol Allergy, 2020, 34(1): 59-69. doi: 10.1177/1945892419871798
[29] Jiang XG, Yang XD, Lv Z, et al. Elevated serum levels of TNF-α, IL-8, and ECP can be involved in the development and progression of bronchial asthma[J]. J Asthma, 2018, 55(2): 111-118. doi: 10.1080/02770903.2017.1318141
[30] Marc-Malovrh M, Camlek L, Skrgat S, et al. Elevated eosinophils, IL5 and IL8 in induced sputum in asthma patients with accelerated FEV1 decline[J]. Respir Med, 2020, 162: 105875. doi: 10.1016/j.rmed.2020.105875
[31] Shute JK, Vrugt B, Lindley IJ, et al. Free and complexed interleukin-8 in blood and bronchial mucosa in asthma[J]. Am J Respir Crit Care Med, 1997, 155(6): 1877-1883. doi: 10.1164/ajrccm.155.6.9196089
[32] Keglowich L, Roth M, Philippova M, et al. Bronchial smooth muscle cells of asthmatics promote angiogenesis through elevated secretion of CXC-chemokines(ENA-78, GRO-alpha, and IL-8)[J]. PLoS One, 2013, 8(12): e81494. doi: 10.1371/journal.pone.0081494
[33] Govindaraju V, Michoud MC, Al-Chalabi M, et al. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration[J]. Am J Physiol Cell Physiol, 2006, 291(5): C957-C965. doi: 10.1152/ajpcell.00451.2005
[34] Pham DL, Ban GY, Kim SH, et al. Neutrophil autophagy and extracellular DNA traps contribute to airway inflammation in severe asthma[J]. Clin Exp Allergy, 2017, 47(1): 57-70. doi: 10.1111/cea.12859
[35] Dong T, Santos S, Yang Z, et al. Sputum and salivary protein biomarkers and point-of-care biosensors for the management of COPD[J]. Analyst, 2020, 145(5): 1583-1604. doi: 10.1039/C9AN01704F
[36] Nakamoto K, Watanabe M, Sada M, et al. Pseudomonas aeruginosa-derived flagellin stimulates IL-6 and IL-8 production in human bronchial epithelial cells: A potential mechanism for progression and exacerbation of COPD[J]. Exp Lung Res, 2019, 45(8): 255-266. doi: 10.1080/01902148.2019.1665147
[37] Barnes PJ. Cellular and molecular mechanisms of asthma and COPD[J]. Clin Sci(Lond), 2017, 131(13): 1541-1558. doi: 10.1042/CS20160487
[38] Xu Q, Chen LX, Ran DH, et al. Bombesin receptor-activated protein regulates neutrophil elastase-induced mucin5AC hypersecretion in human bronchial epithelial cells[J]. Exp Cell Res, 2017, 357(2): 145-154. doi: 10.1016/j.yexcr.2017.05.002
[39] Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease[J]. J Allergy Clin Immunol, 2016, 138(1): 16-27. doi: 10.1016/j.jaci.2016.05.011
[40] Wang JM, Xu L, Murphy WJ, et al. IL-8-Induced T-Lymphocyte Migration: Direct as Well as Indirect Mechanisms[J]. Methods, 1996, 10(1): 135-144. doi: 10.1006/meth.1996.0087
[41] Meniailo ME, Malashchenko VV, Shmarov VA, et al. Direct effects of interleukin-8 on growth and functional activity of T lymphocytes[J]. Int Immunopharmacol, 2017, 50: 178-185. doi: 10.1016/j.intimp.2017.06.023
[42] Sarkar A, Hellberg L, Bhattacharyya A, et al. Infection with Anaplasma phagocytophilum activates the phosphatidylinositol 3-Kinase/Akt and NF-κB survival pathways in neutrophil granulocytes[J]. Infect Immun, 2012, 80(4): 1615-1623. doi: 10.1128/IAI.05219-11
[43] Kobayashi SD, Malachowa N, DeLeo FR. Neutrophils and Bacterial Immune Evasion[J]. J Innate Immun, 2018, 10(5/6): 432-441.
[44] Lazaar AL, Miller BE, Tabberer M, et al. Effect of the CXCR2 antagonist danirixin on symptoms and health status in COPD[J]. Eur Respir J, 2018, 52: 180120.
[45] Mahler DA, Huang S, Tabrizi M, et al. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study[J]. Chest, 2004, 126(3): 926-934. doi: 10.1378/chest.126.3.926
[46] Rennard SI, Dale DC, Donohue JF, et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease[J]. Am J Respir Crit Care Med, 2015, 191(9): 1001-1011. doi: 10.1164/rccm.201405-0992OC
-
| 引用本文: | 黎仙, 李景云, 张媛, 等. IL-8在气道慢性炎症中的作用及研究进展[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(12): 1144-1148. doi: 10.13201/j.issn.2096-7993.2021.12.020 |
| Citation: | LI Xian, LI Jingyun, ZHANG Yuan, et al. The role of IL-8 in the chronic airway inflammation and its research progress[J]. J Clin Otorhinolaryngol Head Neck Surg, 2021, 35(12): 1144-1148. doi: 10.13201/j.issn.2096-7993.2021.12.020 |
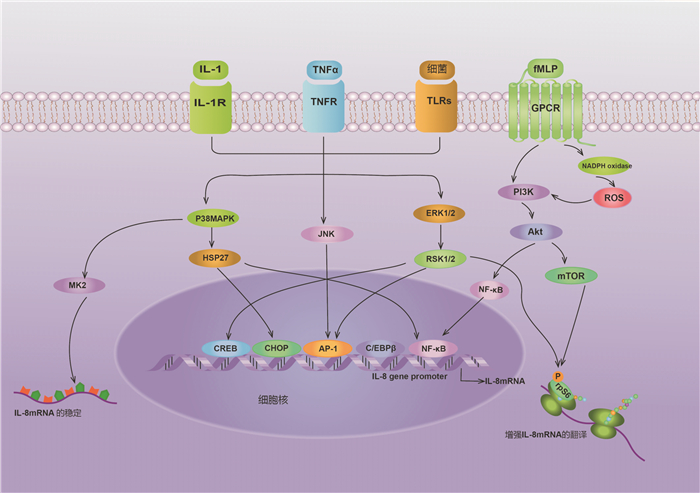
- Figure 1.
- Figure 2.



 下载:
下载: