-
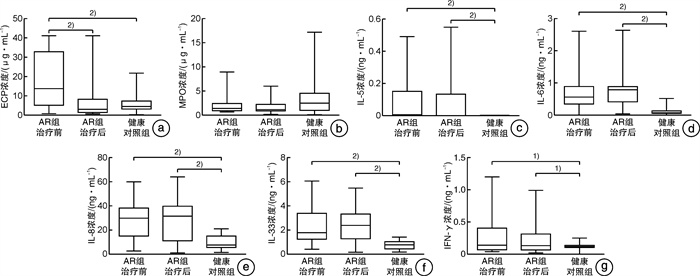
摘要: 目的 探索鼻分泌物中可以辅助诊断变应性鼻炎(AR)以及可用于临床治疗效果评估参考的客观标志物。 方法 纳入33例AR患者(AR组)和21例健康对照者(健康对照组)。收集健康对照及AR患者治疗前后的鼻分泌物,检测鼻细胞学、细胞因子(IL-5、IL-6、IL-8、IL-33、IFN-γ)和炎症因子(ECP、MPO)的浓度,比较健康对照及AR患者治疗前后各指标的差异。分析各指标/各指标差值/各指标差值百分比与临床症状评分/评分差值/评分差值百分比的相关性。 结果 与健康对照组比较,AR组治疗前鼻分泌中的ECP、IL-5、IL-6、IL-8、IL-33和IFN-γ均升高(P < 0.05),MPO未见明显差异;治疗后,AR患者鼻分泌物中的ECP显著下降(P < 0.01),炎症细胞等级及嗜酸粒细胞百分比下降(P < 0.01),但MPO、IL-5、IL-6、IL-8、IL-33和IFN-γ均未见明显改变。治疗前后ECP差值与VAS差值具有相关性(r = 0.348,P = 0.047);IL-5差值与VAS差值(r = 0.406,P = 0.019)和流涕差值(r = 0.429,P = 0.013)具有相关性;鼻分泌物嗜酸粒细胞百分比差值与鼻塞差值具有相关性(r = 0.383,P = 0.028);嗜酸粒细胞百分比与分级乘积差值与VAS评分(r = 0.417,P = 0.016)和鼻塞差值(r = 0.519,P = 0.002)具有相关性;IFN-γ差值百分比与VAS评分/流涕/喷嚏/鼻部总评分差值百分比具有相关性。 结论 鼻分泌物中ECP、IL-6、IL-8、IL-33因子有望成为辅助诊断AR的客观指标,其中ECP、IL-5、IFN-γ、嗜酸粒细胞百分比与分级乘积有望成为判断患者治疗后症状改善的客观指标。Abstract: Objective This study aimed to explore the biomarkers in nasal secretion that can assist in the diagnosis of allergic rhinitis(AR) and can be used to evaluate the therapeutic effect of AR. Methods Thirty-three patients with AR and 21 healthy controls were included. The nasal secretion of healthy controls and patients with AR(before and after treatment) were collected. The cytology, the concentrations of cytokines(IL-5, IL-6, IL-8, IL-33, IFN-γ) and inflammatory mediators(ECP, MPO) were detected. Then, we compared the differences of various biomarkers between healthy controls and AR patients(before and after treatment group). And analyzed the correlation between each biomarkers/biomarkers difference value/the percentage of biomarkers difference value and clinical symptom score/ score difference value / the percentage of score difference value. Results Compared with normal controls, the levels of ECP, IL-5, IL-6, IL-8, IL-33 and IFN-γ in nasal secretion of AR patients were significantly higher than those of normal controls(P < 0.05). There was no significant difference in MPO. After treatment, ECP decreased significantly(P < 0.01), inflammatory cell grade and eosinophil percentage are also decreased(P < 0.01). However, MPO, IL-5, IL-6, IL-8, IL-33 and IFN-γ did not change significantly. The difference value of ECP before and after treatment was correlated with the difference value of VAS score(r = 0.348, P = 0.047). The difference value of IL-5 was correlated with the difference value of VAS score and rhinorrhea, the correlation coefficients were 0.406(P = 0.019) and 0.429(P = 0.013), respectively. The difference value of eosinophil percentage in nasal secretion before and after treatment was correlated with nasal congestion, and the correlation coefficient was 0.383. The difference value of eosinophil percentage multiplied by inflammatory cell grade before and after treatment was correlated with VAS score(r = 0.417, P = 0.016) and nasal congestion difference value(r = 0.519, P = 0.002). The percentage of difference value of IFN-γ before and after treatment was correlated with the percentage of difference value of VAS score / rhinorrhea / sneeze / total nasal symptom score. Conclusion ECP, IL-6, IL-8 and IL-33 in nasal secretion are expected to be objective biomarkers for auxiliary diagnosis of AR. And ECP, IL-5, IFN-γ, eosinophil percentage multiplied by grade is expected to be an objective index to judge the improvement of patients' symptoms after treatment.
-
Key words:
- rhinitis, allergic /
- cytokines /
- inflammatory mediators
-

-
表 1 AR组治疗前后VAS和鼻部症状评分
症状评分 AR组治疗前 AR组治疗后 P VAS 69.70±16.60 21.21±16.61 < 0.0001 鼻塞 1.94±0.85 0.55±0.66 < 0.0001 鼻痒 1.48±0.74 0.33±0.53 < 0.0001 喷嚏 1.70±0.80 0.55±0.61 < 0.0001 流涕 1.79±0.59 0.48±0.56 < 0.0001 TNSS 6.91±2.02 1.92±1.54 < 0.0001 表 2 AR患者治疗前各因子与各症状评分相关性分析
因子 VAS 鼻塞 鼻痒 流涕 喷嚏 TNSS ECP 0.027 -0.089 0.082 -0.329 -0.334 -0.312 MPO 0.131 0.222 -0.054 -0.024 -0.202 -0.077 IL-5 0.240 0.236 0.034 0.236 0.103 0.249 IL-6 -0.076 -0.054 -0.275 -0.148 -0.170 -0.208 IL-8 -0.289 -0.140 -0.037 -0.304 -0.025 -0.201 IL-33 -0.032 -0.050 -0.145 0.110 -0.110 -0.040 IFN-γ -0.101 -0.186 0.167 0.030 0.064 0.058 EOS% 0.059 0.3601) 0.203 -0.004 0.058 0.276 1)P < 0.05。 表 3 AR患者治疗前后各因子变化差值与各症状评分变化差值相关性分析
各因子差值 VAS差值 鼻塞差值 鼻痒差值 流涕差值 喷嚏差值 TNSS差值 ECP差值 0.3481) 0.213 0.064 -0.011 -0.141 0.053 MPO差值 0.291 0.030 0.040 -0.053 -0.100 -0.035 IL-5差值 0.4061) 0.055 0.257 0.4291) 0.183 0.322 IL-6差值 0.128 0.007 -0.041 0.254 0.105 0.125 IL-8差值 -0.070 -0.109 -0.261 -0.112 -0.091 -0.124 IL-33差值 0.009 0.002 0.101 0.287 0.108 0.194 IFN-γ差值 0.179 -0.114 -0.013 0.254 0.150 0.143 EOS%差值 0.312 0.3831) 0.050 0.276 0.270 0.354 EOS%×等级差值 0.4171) 0.5192) 0.097 0.043 0.056 0.224 1)P < 0.05,2)P < 0.01。 表 4 AR患者治疗前后各因子变化百分比与各症状评分变化差值百分比之间相关性分析
各因子差值百分比 VAS差值百分比 鼻塞差值百分比 鼻痒差值百分比 流涕差值百分比 喷嚏差值百分比 TNSS差值百分比 ECP差值百分比 0.334 0.328 -0.176 0.205 -0.067 0.141 MPO差值百分比 -0.034 -0.021 -0.118 -0.135 -0.226 -0.233 IL-5差值百分比 0.255 -0.017 0.264 0.258 0.109 0.232 IL-6差值百分比 -0.141 -0.033 -0.056 0.159 -0.060 -0.025 IL-8差值百分比 0.238 0.288 -0.069 0.111 0.015 0.146 IL-33差值百分比 -0.152 -0.062 0.294 0.123 0.038 0.111 IFN-γ差值百分比 0.4552) 0.217 0.21 0.5072) 0.4021) 0.5162) EOS%差值百分比 0.272 0.111 -0.006 0.226 0.201 0.216 EOS%×等级差值百分比 0.293 0.121 -0.019 0.231 0.238 0.230 1)P < 0.05,2)P < 0.01。 -
[1] Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma(ARIA)2008 update(in collaboration with the World Health Organization, GA(2) LEN and AllerGen)[J]. Allergy, 2008, 63 Suppl 86: 8-160.
[2] Bousquet J, Neukirch F, Bousquet PJ, et al. Severity and impairment of allergic rhinitis in patients consulting in primary care[J]. J Allergy Clin Immunol, 2006, 117(1): 158-162. doi: 10.1016/j.jaci.2005.09.047
[3] Bro ek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma(ARIA)guidelines-2016 revision[J]. J Allergy Clin Immunol, 2017, 140(4): 950-958. doi: 10.1016/j.jaci.2017.03.050
[4] Persi L, Demoly P, Harris AG, et al. Comparison between nasal provocation tests and skin tests in patients treated with loratadine and cetirizine[J]. J Allergy Clin Immunol, 1999, 103(4): 591-594. doi: 10.1016/S0091-6749(99)70229-0
[5] Ciebiada M, Górski P, Antczak A. Evaluation of eicosanoids in nasal lavage as biomarkers of inflammation in patients with allergic rhinitis[J]. Arch Med Sci, 2014, 10(6): 1123-1128.
[6] Diamant Z, Boot JD, Mantzouranis E, et al. Biomarkers in asthma and allergic rhinitis[J]. Pulm Pharmacol Ther, 2010, 23(6): 468-481.
[7] Kim SI, Kwon OE, Park JM, et al. Correlation of Nasal Fluid Biomarkers and Symptoms in Patients with Persistent Allergic Rhinitis[J]. Ann Otol Rhinol Laryngol, 2020, 129(6): 542-547. doi: 10.1177/0003489419898717
[8] Han MW, Kim SH, Oh I, et al. Serum IL-1β can be a biomarker in children with severe persistent allergic rhinitis[J]. Allergy Asthma Clin Immunol, 2019, 15: 58. doi: 10.1186/s13223-019-0368-8
[9] König K, Klemens C, Eder K, et al. Cytokine profiles in nasal fluid of patients with seasonal or persistent allergic rhinitis[J]. Allergy Asthma Clin Immunol, 2015, 11(1): 26. doi: 10.1186/s13223-015-0093-x
[10] Klimek L, Wolf H, Mewes T, et al. The effect of short-term immunotherapy with molecular standardized grass and rye allergens on eosinophil cationic protein and tryptase in nasal secretions[J]. J Allergy Clin Immunol, 1999, 103(1 Pt 1): 47-53.
[11] Klimek L, Dormann D, Jarman ER, et al. Short-term preseasonal birch pollen allergoid immunotherapy influences symptoms, specific nasal provocation and cytokine levels in nasal secretions, but not peripheral T-cell responses, in patients with allergic rhinitis[J]. Clin Exp Allergy, 1999, 29(10): 1326-1335. doi: 10.1046/j.1365-2222.1999.00651.x
[12] Greiff L, Andersson M, Svensson C, et al. Effects of orally inhaled budesonide in seasonal allergic rhinitis[J]. Eur Respir J, 1998, 11(6): 1268-1273. doi: 10.1183/09031936.98.11061268
[13] Kita H, Jorgensen RK, Reed CE, et al. Mechanism of topical glucocorticoid treatment of hay fever: IL-5 and eosinophil activation during natural allergen exposure are suppressed, but IL-4, IL-6, and IgE antibody production are unaffected[J]. J Allergy Clin Immunol, 2000, 106(3): 521-529. doi: 10.1067/mai.2000.108430
[14] Andrews CP, Mohar D, Salhi Y, et al. Efficacy and safety of twice-daily and once-daily olopatadine-mometasone combination nasal spray for seasonal allergic rhinitis[J]. Ann Allergy Asthma Immunol, 2020, 124(2): 171-178. e2. doi: 10.1016/j.anai.2019.11.007
[15] Mullol J, Izquierdo I, Okubo K, et al. Clinically relevant effect of rupatadine 20 mg and 10 mg in seasonal allergic rhinitis: a pooled responder analysis[J]. Clin Transl Allergy, 2019, 9: 50. doi: 10.1186/s13601-019-0293-4
[16] 中华耳鼻咽喉头颈外科杂志编辑委员会鼻科组, 中华医学会耳鼻咽喉头颈外科学分会鼻科学组. 变应性鼻炎诊断和治疗指南(2015年, 天津)[J]. 中华耳鼻咽喉头颈外科杂志, 2016, 51(1): 6-23. doi: 10.3760/cma.j.issn.1673-0860.2016.01.004
[17] Chen J, Zhou Y, Zhang L, et al. Individualized Treatment of Allergic Rhinitis According to Nasal Cytology[J]. Allergy Asthma Immunol Res, 2017, 9(5): 403-409. doi: 10.4168/aair.2017.9.5.403
[18] Chen ST, Sun HL, Lu KH, et al. Correlation of immunoglobulin E, eosinophil cationic protein, and eosinophil count with the severity of childhood perennial allergic rhinitis[J]. J Microbiol Immunol Infect, 2006, 39(3): 212-218.
[19] Klemens C, Rasp G, Jund F, et al. Mediators and cytokines in allergic and viral-triggered rhinitis[J]. Allergy Asthma Proc, 2007, 28(4): 434-441. doi: 10.2500/aap.2007.28.3017
[20] 余文婷, 周玥, 檀慧芳, 等. 血液和鼻分泌物中嗜酸粒细胞和嗜酸粒细胞阳离子蛋白对变应性鼻炎的辅助诊断价值[J]. 临床耳鼻咽喉头颈外科杂志, 2019, 33(11): 1027-1030. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH201911006.htm
[21] Leung DY. Immunologic basis of chronic allergic diseases: clinical messages from the laboratory bench[J]. Pediatr Res, 1997, 42(5): 559-568. doi: 10.1203/00006450-199711000-00001
[22] Ciebiada M, Górski P, Antczak A. Evaluation of eicosanoids in nasal lavage as biomarkers of inflammation in patients with allergic rhinitis[J]. Arch Med Sci, 2014, 10(6): 1123-1128.
[23] 邱昌余, 崔欣燕. 固有免疫和适应性免疫在变应性鼻炎发病中的作用机制[J]. 临床耳鼻咽喉头颈外科杂志, 2019, 33(1): 28-35. https://www.cnki.com.cn/Article/CJFDTOTAL-LCEH201901007.htm
[24] Saito H, Hatake K, Dvorak AM, et al. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins[J]. Proc Natl Acad Sci U S A, 1988, 85(7): 2288-2292. doi: 10.1073/pnas.85.7.2288
[25] Parronchi P, De Carli M, Manetti R, et al. IL-4 and IFN(alpha and gamma)exert opposite regulatory effects on the development of cytolytic potential by Th1 or Th2 human T cell clones[J]. J Immunol, 1992, 149(9): 2977-2983.
[26] Clutterbuck EJ, Hirst EM, Sanderson CJ. Human interleukin-5(IL-5) regulates the production of eosinophils in human bone marrow cultures: comparison and interaction with IL-1, IL-3, IL-6, and GMCSF[J]. Blood, 1989, 73(6): 1504-1512. doi: 10.1182/blood.V73.6.1504.1504
[27] Walsh GM, Hartnell A, Wardlaw AJ, et al. IL-5 enhances the in vitro adhesion of human eosinophils, but not neutrophils, in a leucocyte integrin(CD11/18)-dependent manner[J]. Immunology, 1990, 71(2): 258-265.
[28] Lopez AF, Sanderson CJ, Gamble JR, et al. Recombinant human interleukin 5 is a selective activator of human eosinophil function[J]. J Exp Med, 1988, 167(1): 219-224. doi: 10.1084/jem.167.1.219
[29] Hirai K, Yamaguchi M, Misaki Y, et al. Enhancement of human basophil histamine release by interleukin 5[J]. J Exp Med, 1990, 172(5): 1525-1528. doi: 10.1084/jem.172.5.1525
[30] Xu G, Zhang L, Wang DY, et al. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells[J]. Allergy, 2010, 65(5): 581-589. doi: 10.1111/j.1398-9995.2009.02252.x
[31] Kamekura R, Kojima T, Takano K, et al. The role of IL-33 and its receptor ST2 in human nasal epithelium with allergic rhinitis[J]. Clin Exp Allergy, 2012, 42(2): 218-228. doi: 10.1111/j.1365-2222.2011.03867.x
[32] Ziegler SF, Roan F, Bell BD, et al. The biology of thymic stromal lymphopoietin(TSLP)[J]. Adv Pharmacol, 2013, 66: 129-155.
[33] Klemens C, Rasp G, Jund F, et al. Mediators and cytokines in allergic and viral-triggered rhinitis[J]. Allergy Asthma Proc, 2007, 28(4): 434-441. doi: 10.2500/aap.2007.28.3017
[34] Finkelman FD, Morris SC. Development of an assay to measure in vivo cytokine production in the mouse[J]. Int Immunol, 1999, 11(11): 1811-1818. doi: 10.1093/intimm/11.11.1811
[35] Wagenmann M, Schumacher L, Bachert C. The time course of the bilateral release of cytokines and mediators after unilateral nasal allergen challenge[J]. Allergy, 2005, 60(9): 1132-1138. doi: 10.1111/j.1398-9995.2005.00867.x
[36] Bez C, Schubert R, Kopp M, et al. Effect of anti-immunoglobulin E on nasal inflammation in patients with seasonal allergic rhinoconjunctivitis[J]. Clin Exp Allergy, 2004, 34(7): 1079-1085. doi: 10.1111/j.1365-2222.2004.01998.x
[37] Lou H, Huang Y, Ouyang Y, et al. Artemisia annua-sublingual immunotherapy for seasonal allergic rhinitis: A randomized controlled trial[J]. Allergy, 2020, 75(8): 2026-2036. doi: 10.1111/all.14218
[38] Kim SI, Kwon OE, Park JM, et al. Correlation of Nasal Fluid Biomarkers and Symptoms in Patients with Persistent Allergic Rhinitis[J]. Ann Otol Rhinol Laryngol, 2020, 129(6): 542-547.
-

| 引用本文: | 祝婉婷, 余文婷, 高培, 等. 变应性鼻炎局部炎症客观指标初探[J]. 临床耳鼻咽喉头颈外科杂志, 2021, 35(4): 306-311. doi: 10.13201/j.issn.2096-7993.2021.04.005 |
| Citation: | ZHU Wanting, YU Wenting, GAO Pei, et al. A preliminary study on the biomarkers of local inflammation in allergic rhinitis[J]. J Clin Otorhinolaryngol Head Neck Surg, 2021, 35(4): 306-311. doi: 10.13201/j.issn.2096-7993.2021.04.005 |
- Figure 1.



 下载:
下载: